Low Grade Central Osteosarcoma-A Diagnostic Challenge
Parampalli Srinivas Srilatha1, Ranjini Kudva2, Simanchal P Mohanty3
1 Associate Professor, Department of Pathology, Kasturba Medical College, Manipal, Karnataka, India.
2 Professor, Department of Pathology, Kasturba Medical College, Manipal, Karnataka, India.
3 Professor, Department of Orthopaedics, Kasturba Medical College, Manipal, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Parampalli Srinivas Srilatha, Associate Professor, Department of Pathology, Kasturba Medical College, Manipal-576104, Karnataka, India.
E-mail: srilatha.ps@manipal.edu
Low Grade Central Osteosarcoma (LGCO) is a rare intramedullary and well differentiated variant of osteosarcoma with a better prognosis than the more common conventional variant. It was first described by Unni et al., in 1977. Due to its subtle histological features of malignancy, it is difficult to diagnose on biopsy. Even in the resection specimen it can be mistaken for lesions like fibrous dysplasia, desmoplastic fibroma, parosteal osteosarcoma and fibrosarcoma. Adequate sampling of the tumour is essential to arrive at a correct diagnosis. Histological features such as cytological atypia, mitotic activity, permeation into the bone marrow, entrapment of the native bone, cortical disruption and soft tissue extension will help in the diagnosis of this tumour. We report a case of a 30-year-old man who presented with pain in the right hip of three months duration. On radiological evaluation, a lytic lesion was noted in the upper part of right femur and a possible diagnosis of locally aggressive giant cell tumour of bone was proposed. On histopathological study of the resected upper part of the right femur, a diagnosis of LGCO was rendered.
Bone tumour, Intramedullary, Well differentiated
Case Report
A 30-year-old male presented with pain in the right hip since three months. He had difficulty in walking and getting up from sitting position. He gave history of trauma six months back. There was no history of fever. On local examination of right hip, tenderness was evident over upper part of thigh. No scars or sinuses were noted on the skin. Right gluteal tuberosity was at a higher level than the left side. He had a right lower limb antalgic gait. Systemic examination was within normal limits.
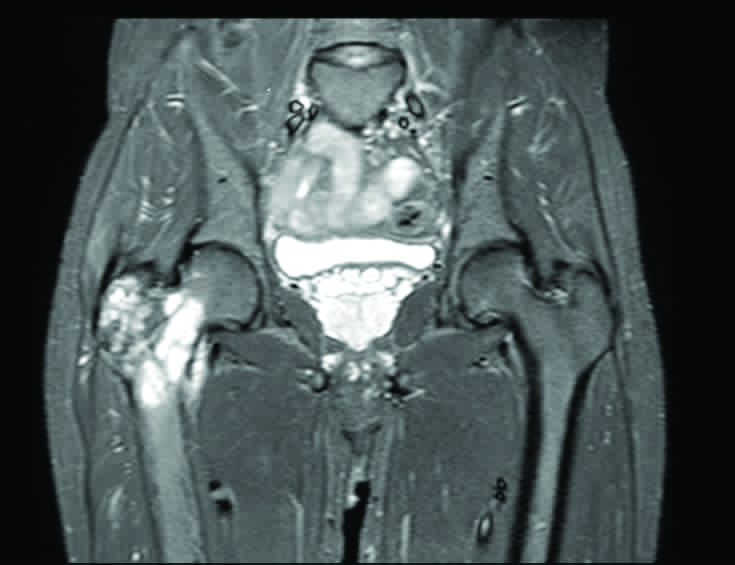
On Magnetic Resonance Imaging (MRI), a well-defined, expansile, lobulated, intramedullary T2 weighted and STIR (short T1 inversion recovery) heterogeneously hyperintense and T1 weighted isointense lesion was noted involving the metaphysis of the neck, lesser and greater trochanter of the right femur. Thinning of the overlying cortex was seen with no break. It showed post contrast diffuse enhancement with foci of blooming [Table/Fig-1]. A possibility of locally aggressive giant cell tumour of right proximal femur was proposed.
MRI image showing a well-defined expansile lobulated intramedullary tumour in the upper part of right femur.
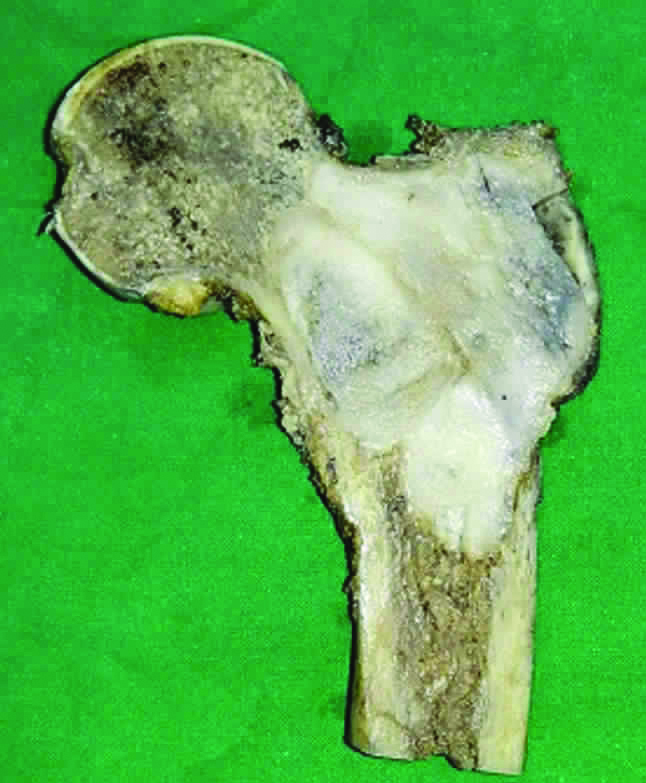
The patient underwent surgical resection prosthesis of right proximal femur and the resected proximal femur was sent for histopathology. On gross examination, the cut surface of the bone showed a circumscribed, expansile grey white lesion measuring 6.5X5.5 cm occupying the metaphyseal region of neck, lesser and greater trochanter. The cortex and periosteum overlying the tumour appeared thinned out [Table/Fig-2].
Gross image of the cut section of resected upper part of right femur showing the circumscribed expansile grey white tumour.
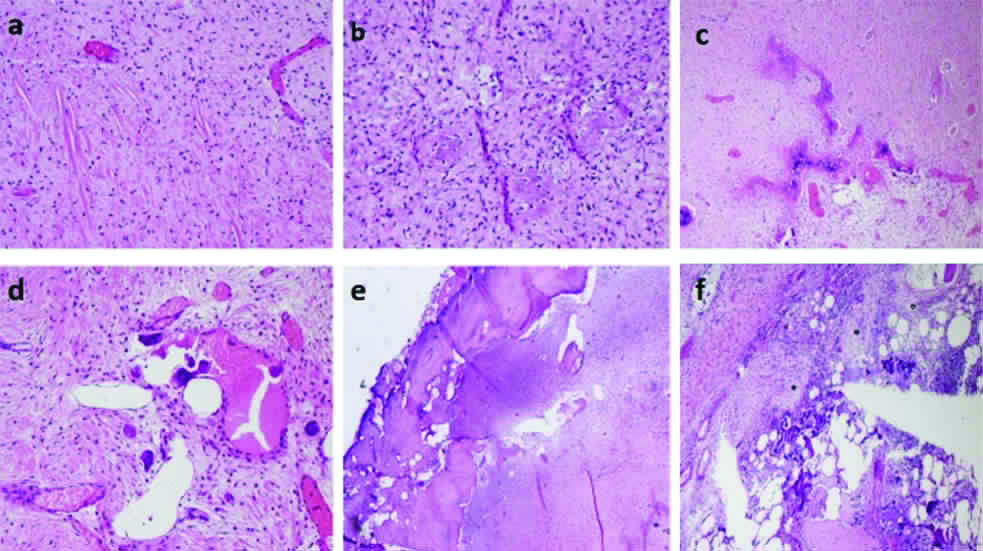
Multiple sections were studied from the lesion, showed a focally hypocellular to moderately cellular tumour comprised of spindle cells arranged in fascicles [Table/Fig-3a]. The spindle cells showed mild to focally moderate nuclear atypia and rare mitotic figure. The stroma was densely collagenous with foci of osteoid production [Table/Fig-3b], focally forming immature bone, focal myxoid areas, focal irregular anastomosing bony trabeculae [Table/Fig-3c] and few aggregates of osteoclastic giant cells [Table/Fig-3d]. The tumour was permeating the inner part of the cortex causing focal thinning of cortex [Table/Fig-3e] and also seen to infiltrate the subjacent marrow spaces in the medullary cavity [Table/Fig-3f]. Both the resected margins were free. A diagnosis of LGCO was established.
Histopathological images of the tumour showing: (a) Moderately cellular areas (H&E;200X); (b) Osteoid production (H&E;200X); (c) Irregular anastomosing bony trabeculae (H&E;100X); (d) Focal aggregate of osteoclastic giant cells (H&E;200X); (e) Permeation into the inner aspect of cortex (H&E;100X); (f) Infiltration into the subjacent marrow spaces (H&E;100X).
Discussion
LGCO is an unusual variant of osteosarcoma with a better prognosis than the common conventional variant. It is an intramedullary bone forming well differentiated malignancy [1]. It was first described in1977 by Unni KK et al., [2]. It has an incidence of 1-2% among the variants of skeletal osteosarcoma. It has been observed in the age group between 18 and 45 years and occurs equally in both the genders [3]. It is more commonly seen in the distal femur and proximal tibia. Uncommon locations being humerus, radius, ulna, fibula, jaw bone, small bones of hand and feet, skull, rib and vertebrae [1,3-5]. In the long bone, it arises from the metaphysis or diametaphysis and rarely from diaphysis [3].
Radiologically, LGCO can be mistaken for aneurysmal bone cyst, osteoblastoma, desmoplastic fibroma, fibrous dysplasia and non-ossifying fibroma [2]. Andersen KJ et al., recognized four radiographic patterns of LGCO: (1) Lytic with varying amounts of coarse and thick trabeculation; (2) Predominantly lytic with few thin incomplete trabeculae; (3) Densely sclerotic; and (4) Mixed, lytic and sclerotic [6,7]. In our case, the tumour was predominantly lytic with few thin trabeculation.
Grossly, LGCO are large grey white, firm, gritty tumours ranging from 2-25 cm and arising within the intramedullary cavity and may breach the cortex with extension into the surrounding soft tissue [3,8,9]. Histologically, this tumour is characterised by a varied pattern of growth ranging from fibroblast-like areas, areas with osteoid matrix and areas with well-formed dense bone formation. The proportion of these areas can vary. Fibroblast-like areas are usually hypocellular to moderately cellular with spindle cells arranged as intersecting fascicles in a collagenous background. These cells show mild atypia and few to rare mitotic figures. The tumour produces variable amount of osteoid matrix. It can form heavy bony trabaculae similar to a parosteal osteosarcoma or can have thin osteoid seams like that of a fibrous dysplasia [2,3]. In rare cases, a pagetoid like dense sclerotic trabecular bone with irregular mosaic pattern of cement lines has been observed [10]. Areas of osteoclastic giant cells and cartilage differentiation can also be seen. This tumour has a permeative growth pattern into the marrow spaces and also entraps the native bony trabeculae. It can cause localized cortical destruction and infiltrate into the adjacent soft tissue [8].
WHO recommends a two-tier grading system for bone tumours based on cellularity and nuclear atypia. Accordingly, this intramedullary well differentiated osteosarcoma is a low grade tumour which can be treated by wide surgical resection [8]. Prognosis is excellent with 90% survival at five years. Few studies have shown that LGCO can have areas of high grade de-differentiation, more commonly in cases of recurrence. The risk of metastasis of such tumour is high. Righi A et al., have described the importance of detecting such high grade areas and its prognostic association. They found that LGCO with >50% high grade component behaves like a conventional (high grade) osteosarcoma and it needs to be treated like the latter with surgical removal and adjuvant chemotherapy. LGCO with <50% high grade component had a good patient survival with surgical removal of tumour irrespective of the use of adjuvant therapy [11,12].
The differential diagnosis on histology includes fibrous dysplasia, desmoplastic fibroma, parosteal osteosarcoma and fibrosarcoma [Table/Fig-4]. Fibrous dysplasia is a benign lesion which shows no cellular atypia, no mitotic figures and no cortical disruption. Parosteal osteosarcoma has a surface location unlike LGCO which is an intramedullary tumour. Desmoplastic fibroma and fibrosarcoma are spindle cell tumours and show no immature bone formation [2-4,13]. LGCO expresses immunohistochemical markers like Murine Double-Minute Type2 (MDM2) and Cyclin-Dependent Kinase 4 (CDK4). These markers are helpful in distinguishing it from benign histological mimics [14].
Table depicting the clinical, radiological and histological features of differential diagnosis of LGCO.
| Features | Fibrous dysplasia | Desmoplastic fibroma | Fibrosarcoma | Parosteal osteosarcoma | Low grade central osteosarcoma |
|---|
| 1. | Behaviour | Benign spindle cell fibro-osseous lesion | Benign spindle cell tumor | Malignant spindle cell tumour | Malignant low grade bone tumour | Malignant low grade bone tumour |
| 2. | Location | Intramedullary | Intramedullary | Intramedullary | Surface of bone | Intramedullary |
| 3. | Radiology | Well defined radiolucent with sclerotic margins | Well defined radiolucent lesion that may expand host bone | Ill-defined permeative moth eaten appearance | Heavily mineralized mass attached to cortex with broad base and wraps around the involved bone | Lytic or sclerotic or mixed with thin or thick trabeculations |
| 4. | Cortical destruction and soft tissue involvement | Not present | Seen in larger tumours | Present | Can be present | Can be present |
| 5. | Permeative growth pattern in to the marrow spaces | Not present | Can be present | Present | Not present | Present |
| 6. | Presence of bony trabeculae/ immature bone | Branched, delicate, curvilinear bony trabeculae | Not present | Not present | Long parallel well-formed bony trabeculae | Thin osteoid seams or heavy bony trabeculae |
| 7. | Spindle cell cellularity | Hypocellular | Variably cellular in a collagenised stroma | Hypercellular with variable amount of collagen | Hypocellular | Hypocellular to moderately cellular |
| 8. | Cellular atypia | Not present | Minimal/Absent | Present | Mild | Mild |
| 9. | Mitoses | Absent | Rare | Present | Occasional to few | Occasional to few |
Conclusion
Since LGCO has subtle and varied histological features of malignancy, it is difficult to diagnose on biopsy. It is essential to do adequate sampling from multiple foci of the tumour for detailed histological study. Helpful histological features which can lead to the diagnosis of LGCO include cytological atypia, mitotic activity, permeation into bone marrow or native bone, cortical disruption and soft tissue extension. It is important to make a correct diagnosis for appropriate treatment. This is to prevent local recurrence which is often of high grade nature.
[1]. Kim YC, Suh J-S, Kim M, Choo H-J, Huh Y-M, Low-grade osteosarcoma of the spine: a case reportJ Korean Radiol Soc 2007 56:575-78.10.3348/jkrs.2007.56.6.575 [Google Scholar] [CrossRef]
[2]. Unni KK, Dahlin DC, McLeod RA, Pritchard DJ, Intraosseous well differentiated osteosarcomaCancer 1977 40(3):1337-47.10.1002/1097-0142(197709)40:3<1337::AID-CNCR2820400351>3.0.CO;2-X [Google Scholar] [CrossRef]
[3]. Gilg MM, Liegl B, Wibmer C, Maurer-Ertl W, Leithner A, Central low-grade osteosarcoma with an unusual localization in the diaphysis of a 12-year-old patientRadiol Oncol 2013 47(2):192-96.10.2478/raon-2013-001523801917 [Google Scholar] [CrossRef] [PubMed]
[4]. Muramatsu K, Hashimoto T, Seto S, Gondo T, Ihara K, Taguchi T, Low-grade central osteosarcoma mimicking fibrous dysplasia: a report of two casesArch Orthop Trauma Surg 2008 128:11-15.10.1007/s00402-006-0280-917203284 [Google Scholar] [CrossRef] [PubMed]
[5]. Tabatabaei SH, Jahanshahi G, Marvasti FD, Diagnostic challenges of low-grade central osteosarcoma of jaw: A literature reviewJ Dent Shiraz Univ Med Sci 2015 16(2):62-67. [Google Scholar]
[6]. Tang F, Min L, Zhou Y, Luo Y, Tu C, Low-grade central osteosarcoma in proximal humerus: A rare entityOnco Targets and Therapy 2017 10:5165-72.10.2147/OTT.S14281829123414 [Google Scholar] [CrossRef] [PubMed]
[7]. Andresen KJ, Sundaram M, Unni KK, Sim FH, Imaging features of low-grade central osteosarcoma of the long bones and pelvisSkeletal Radiol 2004 33(7):373-79.10.1007/s00256-004-0796-415175837 [Google Scholar] [CrossRef] [PubMed]
[8]. Inwards C, Squire J, Low-grade central osteosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editorsWorld Health Organization Classification of Tumours. Pathology and genetics of tumours of soft tissue and bone 2013 4th edLyonIARC Press:281 [Google Scholar]
[9]. Ostrowski ML, Johnson ME, Smith PD, Chevez-Barrios P, Spjut HJ, Low-Grade Intraosseous Osteosarcoma With Prominent Lymphoid InfiltrateArch Pathol Lab Med 2000 124:868-71. [Google Scholar]
[10]. Franchi A, Bacchini P, Rocca CD, Bertoni F, Central low-grade osteosarcoma with pagetoid bone formation: a potential diagnostic pitfallModern Pathology 2004 17:288-91.10.1038/modpathol.380006914976532 [Google Scholar] [CrossRef] [PubMed]
[11]. Righi A, Paioli A, Dei Tos AP, Gambarotti M, Palmerini E, Cesari M, High-grade focal areas in low-grade central osteosarcoma: high-grade or still low-grade osteosarcoma?Clin Sarcoma Res 2015 5:2310.1186/s13569-015-0038-726524981 [Google Scholar] [CrossRef] [PubMed]
[12]. Klein MJ, Siegal GP, Osteosarcoma: anatomic and histologic variantsAm J Clin Pathol 2006 125:555-81.10.1309/UC6KQHLD9LV2KENN [Google Scholar] [CrossRef]
[13]. Inwards CY, Low-grade central osteosarcoma versus fibrous dysplasiaPathology Case Reviews 2001 6(1):22-27.10.1097/00132583-200101000-00005 [Google Scholar] [CrossRef]
[14]. Yoshida A, Ushiku T, Motoi T, Shibata T, Beppu Y, Fukayama M, Immunohistochemical analysis of MDM2 and CDK4 distinguishes low-grade osteosarcoma from benign mimicsMod Pathol 2010 23(9):1279-88.10.1038/modpathol.2010.12420601938 [Google Scholar] [CrossRef] [PubMed]