Blood glucose test is an important routine test done in biochemistry. Plasma without the use of an antiglycolytic agent is inadequate for glucose estimation unless the delay between collection and processing is minimum i.e., 30 minutes [1]. This is because the cells present in blood, mainly RBCs consume glucose in stored blood by glycolysis at a rate of 5-7% per hour [2].
The mechanism of glucose metabolism is well known, and is caused by the action of various enzymes in the erythrocytes. When conventional antiglycolytic agents like sodium fluoride (NaF) or iodoacetate, is added to the blood sample, it enters the erythrocyte through the cell membrane, inhibits enzymes such as enolase or glyceraldehyde 3 phosphate dehydrogenase respectively, thus inhibiting the metabolism of glucose and maintains the level of glucose in the blood sample. NaF or iodoacetate treated samples are used when delay of an hour or more is unavoidable. NaF is not only the most widely used antiglycolytic agent for preserving glucose but also has been reported to be the best available preservative for glucose estimation [3]. Recently, however, it has been discovered that such conventional methods using NaF or iodoacetate do not always produce a complete inhibition of glycolysis and glucose concentration decreases by 5-15% within the first 2-4 hour after sample collection [4,5]. Besides, they have a tendency to cause haemolysis, and thus are not always suitable for treating valuable blood samples [6]. Also, results for glucose are affected by NaF when unmodified glucose oxidase-peroxidase (GOD-POD) techniques (glucose oxidase is sensitive to inhibitory action of fluoride) are used [7,8]. Both fluoride and iodoacetate do not act on the first step in the pathway by which glucose is consumed. Fluoride acts on an enzyme called enolase which is the 8th enzyme in the glycolytic pathway. Iodoacetate acts on an enzyme called glyceraldehyde 3-phosphate dehydrogenase which is the 5th enzyme in the glycolytic pathway. Thus, theoretically iodoacetate should be able to arrest consumption of glucose earlier than fluoride. However, this does not prevent glucose from entering cells and being phosphorylated by hexokinase and further metabolized to either, the pentose pathway, glycogen or intermediates of the first step of glycolysis. Only after this initial loss of glucose in the first three hours after blood collection, both fluoride and iodoacetate are effective in preserving glucose for at least three days. Further, NaF is not an inhibitor specific to enzymes belonging to the glycolytic pathway, but is a general inhibitor for enzymes like urease [1,9]. Therefore, in many cases, there is an adverse effect on other routine analysis [1,6]. Moreover, NaF inhibits activity of the sodium-potassium-ATPase in the erythrocyte and interferes with correct assessment of electrolytes, sodium and potassium [1]. For estimation of glucose, American Association for Clinical Chemistry (AACC) and American Diabetes Association (ADA) in their most recent guidelines for laboratory analysis in the diagnosis and management of diabetes mellitus, recommends that the plasma tube should be centrifuged within minutes of collection or if not, the tube can be maintained in an ice-slurry for up to 30 minutes before centrifugation [10]. This recommendation is however impractical to follow in clinical practice. ADA/WHO also endorses that NaF is an unreliable antiglycolytic agent, instead citrate should be used. Antiglycolytic action of citrate is by rapid acidification of blood pH below 5.9 which inhibits the first two key enymes (hexokinase and phosphofructokinase) of glycolytic pathway [11]. Citrate tubes specific for glucose estimation is not marketed commercially in India. Juricic G et al., found that liquid citrate buffer not by itself but in combination with NaF/sodium EDTA (available as glucomedic tubes) are most efficient glycolytic inhibitor currently available [12]. Another glycolytic inhibitor, glyceraldehyde has been found to be suitable for other analytes too, however, it is not effective glycolytic inhibitor all by itself. Combination of glyceraldehydes with NaF and potassium oxalate is more effective antiglycolytic agent [13]. It is therefore, desirable to provide a novel glycolysis inhibiting agent which is not only an effective antiglycolytic agent alone but also is safe and simple, causes less damage such as haemolysis to the blood sample, and does not interfere in the estimation of other analytes. D-Mannose is one such antiglycolytic agent. It is a hexose monosaccharide, which competitively inhibits glucokinase as it competes with glucose in the first step of glycolysis [Table/Fig-1]. The agent is believed to prevent entry of glucose into erythrocytes, thus restricting access to glycolytic enzymes. Since it is not an enzyme poison it can be used to preserve samples used for other investigations e.g., urea. D-Mannose has been suggested before to be a useful antiglycolytic agent to preserve glucose in blood samples and reportedly acts faster than NaF [14-16]. In view of the above, this study was undertaken to evaluate the value of D-Mannose as an effective glycolytic inhibitor in isolation.
Materials and Methods
This study was an observational study done to evaluate the role of D-Mannose as an antiglycolytic agent, during October 2017, at Biochemistry laboratory of tertiary care center after institutional ethical committee approval. Samples were collected from healthy individuals who reported for routine annual medical examination. Informed consent was obtained from all individual participants included in the study.
The study was executed in 3 steps: First set of experiment was to determine the optimal concentration of D-mannose. Whole blood samples from 15 individuals included in the study were collected in vacuum evacuated tube with heparin as anticoagulant. Plasma obtained after centrifugation of heparin tubes from each sample was aliquoted into 5 parts of 1 mL each. In one, no additive was added while in others D-mannose was added in concentration of 1,2,3 and 5 mg/mL. Blood glucose was analysed immediately, at 1.5 hours, 3 hours and 5 hours in all the aliquots to check out the optimal concentration of D-mannose.
Second set of experiment was to ascertain any interference caused by D-mannose in estimation of analytes. Blood samples from 15 individuals were collected in plain tube without additive. Serum separated after centrifugation was aliquoted into 2 parts of 1 mL each. In one, D-mannose was added in a conc of 3 mg/mL whereas in the other nothing was added. Blood glucose and other routine analytes (Urea, Creatinine, Protein, Albumin, AST, ALT, ALP, Amylase, LDH, CK, Sodium, Potassium, Calcium, Phosphorus, Cholesterol, Triglycerides, HDL-Cholesterol (HDLc), Bilirubin) were estimated to check whether D-mannose interferes with the estimation of other analytes.
In third set of experiment, comparison of D-mannose with other sample preservatives was made. Samples were collected from 15 individuals in four different preservatives. Potassium EDTA at a concentration of 1.8 mg/mL, Sodium Heparin at a concentration of 143 USP (United States Pharmacopeia) points/mL, D-mannose at a concentration of 3 mg/mL and NaF at a concentration of 1.8 mg/mL. To the sample collected in heparin, D-mannose (in concentration of 3 mg/mL) was added to compare utility of D-mannose against other preservatives (EDTA and NaF). Blood glucose and other routine analytes were measured immediately, at 1.5 hours and 3 hours of sample collection.
All the samples were analysed in Clinical Chemistry Analyser TRANSASIA-ERBA XL-300 using commercially purchased Erba XL System packs. All samples were processed on the same day of collection. D-Mannose used was from Sigma-Aldrich firm (Product no M6020). A total of 19 biochemistry analytes were estimated in all the samples. As per the kit, glucose (GOD-POD method) had an intra assay Co-Efficient of Variation (CV) of 2.5% at level I and 1.0% at level II. The inter assay CV was 2.34 & 1.22 respectively at level I & Level II.
For CV calculation assays were carried out in triplicates.
Statistical Analysis
The difference in the levels of Glucose using various preservatives was evaluated by Student’s t-test and the p-value of <0.05 was considered to be significant.
Results
First experiment was to optimize the concentration of D-mannose [Table/Fig-2,3]. It was observed that at a concentration of 3 mg/mL at the end of 1.5 hours the decrease of glucose was minimum i.e., 4.9 mg/dL, at the end of 3 hours, the decrease was 11.7 mg/dL and at the end of 5 hours, the decrease was least, being 16 mg/dL.
Optimization of concentration of Mannose as an antiglycolytic agent.
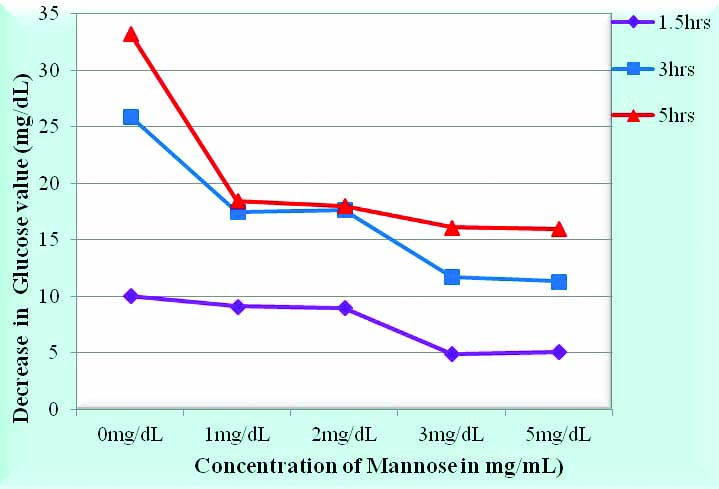
Numerical data for Table/Fig-2 shows the mean decrease in glucose in 15 subjects at 1.5 hrs, 3hrs and 5 hrs from the baseline measured immediately after drawing blood with different concentrations of mannose ie at 0 mg/dL, 1 mg/dL, 2 mg/dL, 3 mg/dL and 5 mg/dL.
| Time & Mannose conc | Mean decrease | Std. Deviation | Coefficient of Variation |
|---|
| 1.5 hrs0 mg/dL | 10.07 | 0.605 | 0.06 |
| 1.5 hrs1 mg/dL | 9.13 | 0.774 | 0.084 |
| 1.5 hrs2 mg/dL | 9 | 1.044 | 0.116 |
| 1.5 hrs3 mg/dL | 4.93 | 1.097 | 0.221 |
| 1.5 hrs5 mg/dL | 5.12 | 1.123 | 0.219 |
| 3 hrs0 mg/dL | 25.93 | 1.23 | 0.047 |
| 3 hrs1 mg/dL | 17.47 | 1.95 | 0.111 |
| 3 hrs2 mg/dL | 17.67 | 2.01 | 0.113 |
| 3 hrs3 mg/dL | 11.73 | 1.98 | 0.168 |
| 3 hrs5 mg/dL | 11.33 | 1.99 | 0.175 |
| 5 hrs0 mg/dL | 33.27 | 3.67 | 0.110 |
| 5 hrs1 mg/dL | 18.47 | 4.192 | 0.226 |
| 5 hrs2 mg/dL | 18 | 4.13 | 0.229 |
| 5 hrs3 mg/dL | 16.13 | 4.312 | 0.267 |
| 5 hrs5 mg/dL | 16 | 3.57 | 0.223 |
No significant interference was observed with D-mannose as a preservative in estimation of routine analytes (i.e., urea, creatinine, uric acid, phosphorus, triglycerides, cholesterol, HDL, creatine kinase, sodium, amylase, protein, albumin, ALT, AST, bilirubin, alkaline phosphatase, calcium, LDL and LDH) except potassium. There was a statistically significant difference (p-value<0.05) between the values of potassium with D-mannose and without D-mannose [Table/Fig-4].
Comparison of serum potassium levels with and without mannose as preservative.
| Analyte | Levels | SD | CV |
|---|
| Potassium with mannose | 4.33 | 0.29 | 0.07 |
| Potassium | 4.46 | 0.35 | 0.08 |
Results of comparative efficacy of D-mannose with regards to EDTA, NaF and without additive (plain tube) showed a decrease of 3 mg/dL in glucose with D-mannose compared with 16 mg/dL with NaF as preservative at the end of 3 hours [Table/Fig-5,6].
Efficacy of Mannose as an antiglycolytic agent compared to other preservatives.
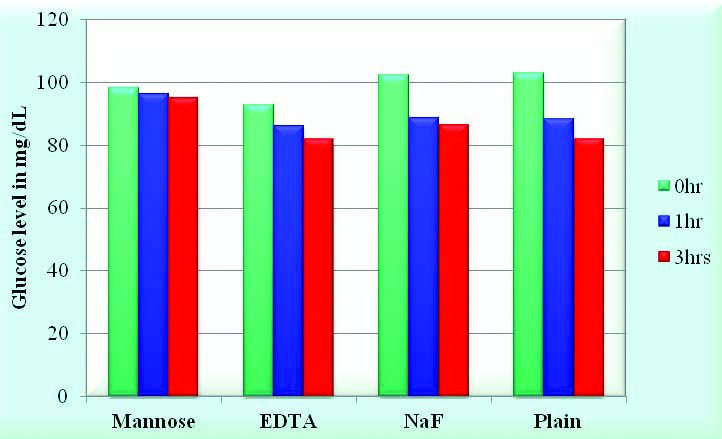
Illustrating the mean values of Glucose in 15 subjects done in the presence of four different preservatives that is Potassium EDTA at a conc of 1.8 mg/mL, Sodium Heparin at a concentration of 143 USP points, Mannose at a concentration of 3 mg/mL & Sodium fluoride at a conc of 1.8 mg/mL.
| Preservative | 0 hr | 1 hr | 3 hr |
|---|
| Mannose | 98.07 | 96.4 | 95 |
| EDTA | 92.67 | 86.13 | 81.73 |
| NaF | 102.27 | 88.6 | 86.34 |
| Plain | 102.8 | 88.47 | 81.93 |
It was observed that the values of creatine kinase were lowest (27, 27, 21 IU/L at the end of 0 hours, 1.5 hours and 3 hours respectively) with EDTA compared to 61, 61, 58 IU/L with D-mannose; 63, 67, 71 IU/L with sodium fluoride and 69, 66, 66 IU/L with plain vacutainer [Table/Fig-7,8].
Effect of different preservatives on Creatine Kinase.
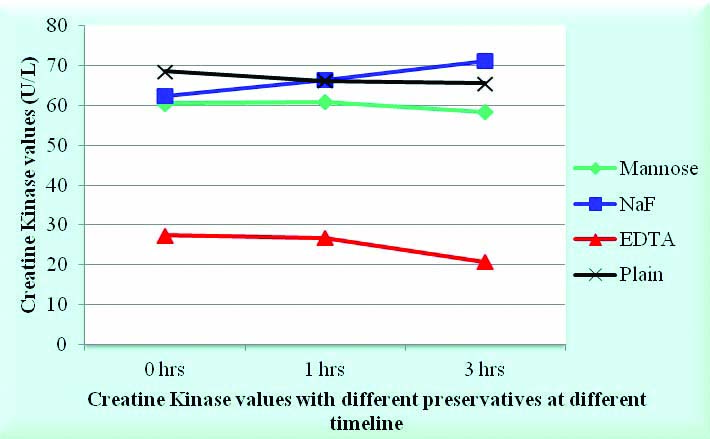
Illustrating the mean values of Creatine Kinase in 15 subjects done in the presence of four different preservatives that is Potassium EDTA at a conc of 1.8 mg/mL, Sodium Heparin at a concentration of 143 USP points, Mannose at a concentration of 3 mg/mL & Sodium fluoride at a conc of 1.8 mg/mL.
| Preservative | 0 hr | 1 hr | 3 hr |
|---|
| Mannose | 60.5 | 61 | 58.38 |
| EDTA | 27.38 | 26.75 | 20.75 |
| NaF | 62.5 | 66.5 | 71.25 |
| Plain | 68.63 | 66.25 | 65.63 |
Discussion
In the first set of experiment, the optimal concentration of D-mannose at which rate of decrease of glucose is minimum was found out to be of 3 mg/mL. At this concentration of D-mannose, the decrease in glucose was least (16 mg/dL) at the end of 5 hours. Chan AYW et al., in their study have quoted a decrease of glucose at the end of 2 hrs with D-mannose at 3 mg/mL of 4.2 mg/dL [17]. In the second set of experiment, various analytes were estimated using D-mannose as a preservative and for comparison the same analytes were measured without any preservative to assess any interference caused by D-mannose in the analysis of the parameters. Burrin JM et al., have proposed that D-mannose interferes with GOD-POD method causing a positive interference [18]. This may be due to the presence of glucose present as a contaminant in the D-mannose used. In our study, the mean glucose with D-mannose was 112±08 mg/dL and that without D-mannose was 108.9±09 mg/dL. There was no statistical significance in the difference between the two (p > 0.05). Thus, our study illustrates the fact that D-mannose does not interfere with GOD-POD method.
The rest of the analytes i.e., urea, creatinine, uric acid, phosphorus, triglycerides, cholesterol, HDL, Creatine kinase, Sodium, Amylase, protein, albumin, ALT, AST, bilirubin, alkaline phosphatase, calcium, LDL and LDH did not show any significant change with D-mannose as a preservative. Thus, D-mannose can be used for estimation of all analytes except potassium. There was a statistically significant difference between the values of potassium with D-mannose and without D-mannose i.e., 4.33±0.29 meq/L and 4.46±0.35 meq/L respectively (p<0.05). Nakashima K et al., have recorded an increase in plasma potassium with D-mannose as a preservative which they attribute to leaking of potassium from residual erythrocytes (ATP produced by glycolysis is utilized to maintain Na+K+ ATPase pump on erythrocytes) as a result of inhibition of glycolysis [14]. D-Mannose can be used for urea estimation with urease method as it does not interfere with urease enzyme unlike NaF. In the third experiment, the comparison of estimations of AST, Creatine kinase, Cholesterol, creatinine, uric acid, protein and glucose was done with three different preservatives i.e., NaF at a concentration of 2 mg/mL, Potassium EDTA with 3 mg/mL and heparin with sodium heparin 143 USP units at 0 hours, 1.5 hours and 3 hours. With D-mannose as preservative, our study showed a decrease of 3 mg/dL of glucose at the end of 3 hours compared with 16 mg/dL with NaF as preservative. Therefore, D-mannose is a more potent preservative compared to NaF in the first three hours. Van Dijck P et al., too got similar findings with D-mannose compared to NaF, but interfered with Creatine kinase (in the methods involving hexokinase and glucose-6-phosphate dehydrogenase) [19].
In our study, the values of Creatine kinase were observed lower with EDTA at the end of 0 hours, 1.5 hours and 3 hours compared to D-mannose, NaF and plain vacutainer [Table/Fig-7,8]. No other studies are available could be seen with CK levels in EDTA. The other analytes did not show significant change with time with the different preservatives used. This could be attributed to the fact that these analytes are stable upto 24 hours at room temperature.
Conclusion
The results confirmed our objective of proving D-Mannose as an ideal preservative for blood glucose. It also proved its efficacy as it is not an enzyme poison unlike sodium fluoride, thereby giving more constant results in enzyme assays. From our study, it has been seen that D-mannose at a concentration of 3 mg/mL does not interfere with estimations of urea, creatinine, albumin, protein, calcium, phosphorus, ALT, AST, ALP, LDH, Amylase, CPK, uirc acid, cholesterol, triglycerides, HDL, bilirubin and sodium. Therefore, these analytes can be estimated from a single tube containing D-mannose as an antiglycolytic agent.