Introduction
The use of bright field microscopes for assessment of direct clinical specimens dates back to the advent of Microbiology and has had an immense impact on laboratory diagnosis of bacterial, fungal and protozoal diseases. Microscopic examination of clinical specimens not only has the advantage of rapid diagnosis but also adds specific details on the extent of tissue invasion and inflammatory response elicited by micro-organisms. The major limitations of microscopy are subjectivity and the need for high level of diagnostic skill drawn from extensive experience [1].
Bright field microscopes are most commonly used in diagnostic laboratories worldwide. The various advantages of these microscopes are as follows: (1) Ease of use with fewer adjustments required for viewing specimens; (2) Some specimens can be viewed without staining and the optics used in the bright field technique do not alter the colour of the specimen; (3) These are adaptable with new technology and optional pieces of equipment can be implemented with bright field illumination to give versatility in the tasks it can perform. However, bright field microscopy also has certain disadvantages which include very low contrast as a result of which most cells have to be stained to be visualized. Staining may introduce extraneous details into the specimen that should not be present. Also, the user will need to be knowledgeable in proper staining techniques [1].
Clinical Microbiologists often come across strange structures during microscopic examination of clinical specimens. These structures are often confused with and misreported as micro-organisms, thereby, leading to erroneous diagnosis and inappropriate treatment of patients especially in developing countries. Since infections caused by multi-drug resistant micro-organisms are rampant with limited therapeutic options available, it is imperative not to over-report and treat infections. Surprisingly, despite the frequent encounters of this issue in daily practice there is dearth of published literature.
We studied series of cases in a super speciality hospital from January 2015 to December 2016 with the aim of enumerating artefacts and mimickers that may simulate infectious agents. Microscopic examination of various clinical specimens like pus, tissue, sputum and blood (for peripheral smear examination) received by the department of Microbiology during this period was carried out as per requisition received from clinicians. Photographs of several microscopic artefacts were obtained using a high definition camera. For comparative purpose, photographs of common pathogenic micro-organisms closely resembling these structures were also obtained.
Case Series
(a) Artefacts Resembling Fungal Hyphae:
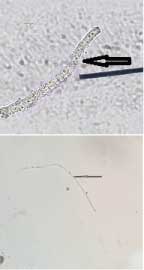
Case 1: KOH mount of skin scrapings obtained from a patient, the findings of which have been shown in [Table/Fig-1]. On first look, it gave an appearance of thin filamentous structures morphologically resembling fungal hyphae. However, upon careful observation it was found to be outline of an epithelial cell.
KOH mount of skin scrapings obtained from a patient (400x).

Case 2: Filamentous structures morphologically resembling fungal hyphae in KOH mount have been depicted in [Table/Fig-2a,b] respectively. These were in fact exogenous fibres with blunt ends and varying thickness.
a) Filamentous structures morphologically resembling fungal hyphae in KOH mount (400x); b) Filamentous structures morphologically resembling fungal hyphae in KOH mount (400x).
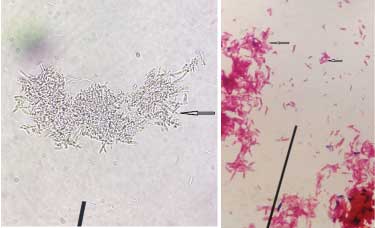
Case 3: Microscopic findings of KOH mount of debrided wound tissue sample showing filamentous structures of varying thickness arranged in clumps morphologically resembling mycelia have been shown in [Table/Fig-3a,b] depict Gram stained smear of the same sample showing thick filamentous Gram-negative structures arranged singly, in palisades and clumps. Both aerobic bacterial & fungal cultures of this tissue sample yielded moist grey colonies which were subsequently confirmed to be bacterial in origin by microscopic examination of Gram stained colony smears.
a) KOH mount of debrided wound tissue sample showing filamentous structures of varying thickness arranged in clumps morphologically resembling mycelia (400x); b) Gram stained smear of wound tissue sample showing thick filamentous Gram negative structures arranged singly, in palisades & clumps (1000x).
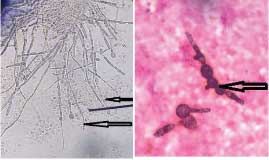
Case 4: KOH mount and Gram stained smear of nail scrapings of a patient were prepared and observed microscopically, the findings of which have been depicted in [Table/Fig-4a,b] respectively. After being initially identified as fungal hyphae, these were later correctly interpreted as budding yeast cells with pseudohyphae displaying variable width with irregular constriction and budding.
a) KOH mount of nail scrapings of a patient showing budding yeast cells with pseudohyphae (400x); b) Gram stained smear of nail scrapings of a patient showing budding yeast cells with pseudohyphae (1000x).
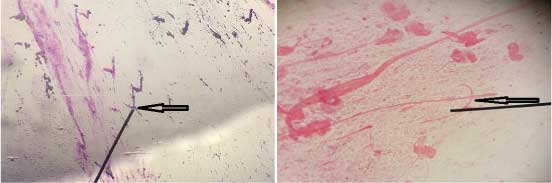
Case 5: Gram stained smears of a patient’s sputum sample showing mucus threads resembling fungal hyphae have been shown in [Table/Fig-5a,b].
a) Gram stained smear of a patient’s sputum sample showing mucus threads resembling fungal hyphae (1000x); b) Gram stained smear of a patient’s sputum sample showing mucus threads resembling fungal hyphae (1000x).
KOH mount and Gram stained smears of septate, branching fungal hyphae have respectively been depicted in [Table/Fig-6,7a,b] respectively.
Gram stained smear showing septate, branching fungal hyphae (1000x).

a) KOH mount showing septate, branching fungal hyphae (400x); b) KOH mount showing septate, branching fungal hyphae (400x).
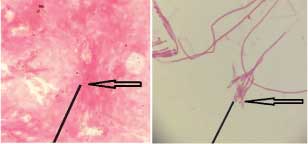
(b) Artefacts Resembling Microfilariae:
Case 1: Leishman stained peripheral smear of blood showing a structure morphologically resembling microfilaria has been depicted in [Table/Fig-8]. However, taking certain parameters like size, thickness, rough & non-uniform borders and absence of nuclear structures, this smear was reported as negative.
Leishman stained peripheral smear of blood showing a structure morphologically resembling microfilaria (1000x).
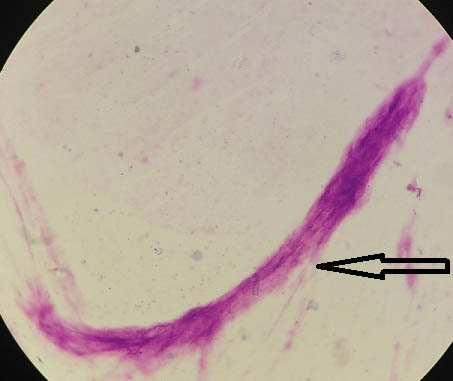
[Table/Fig-9] depicts Leishman stained peripheral blood smears showing sheathed microfilaria of Wuchereria bancrofti under oil immersion (1000x). No nuclei in the tail tip were noted.
Leishman stained peripheral blood smears showing sheathed microfilaria of Wuchereria bancrofti (400x).
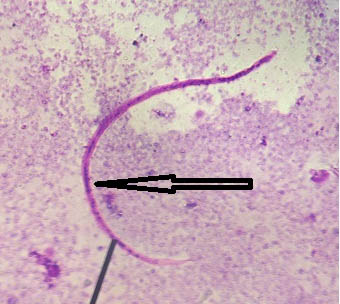
(c) Artefacts Resembling Malaria Parasites:
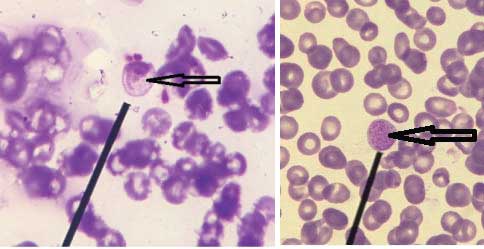
Case 1: Leishman stained peripheral smear of blood showing an artefact morphologically resembling ring stage of Plasmodium spp. has been depicted in [Table/Fig-10].
Leishman stained peripheral smear of blood showing an artefact morphologically resembling ring stage of Plasmodium spp. (1000x).
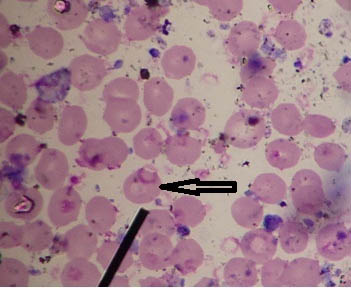
Case 2: Leishman stained peripheral smears of blood showing granulocytes, platelet clumps and reticulocytes which were misidentified as schizonts of Plasmodium spp. have been depicted in [Table/Fig-11a-c] respectively.
a) Leishman stained peripheral smear of blood showing granulocytes misidentified as schizonts of Plasmodium spp. (1000x); b) Leishman stained peripheral smear of blood showing platelet clumps misidentified as schizonts of Plasmodium spp. (1000x); c) Leishman stained peripheral smear of blood showing reticulocytes misidentified as schizonts of Plasmodium spp. (1000x).
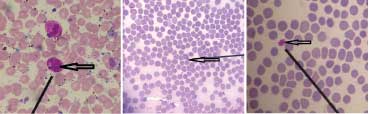
Case 3: Monocytes and lymphocytes, which closely resembled and therefore confused with gametocytes of Plasmodium vivax have been depicted in [Table/Fig-12a,b] respectively.
a) Leishman stained peripheral smear of blood showing monocytes misidentified as gametocytes of Plasmodium vivax (1000x); b) Leishman stained peripheral smear of blood showing lymphocytes misidentified as gametocytes of Plasmodium vivax (1000x).
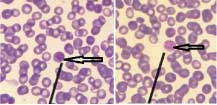
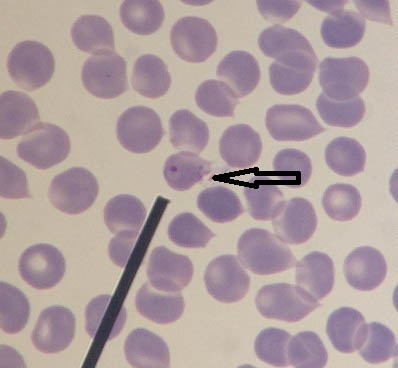
[Table/Fig-13] shows ring stage of Plasmodium spp. with prominent dot-like eosinophilic nuclear chromatin and ring-like basophilic cytoplasm. Schizont stage of Plasmodium spp. showing merozoites has been depicted in [Table/Fig-14a,b] respectively. Gametocyte stage of Plasmodium vivax showing condensed eosinophilic nuclear chromatin has been depicted in [Table/Fig-15a,b] respectively.
Leishman stained peripheral smear of blood showing ring stage of Plasmodium spp. (1000x).
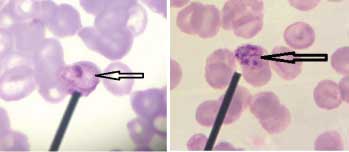
a) Leishman stained peripheral smear of blood showing schizont stage of Plasmodium spp. showing merozoites (1000x); b) Leishman stained peripheral smear of blood showing schizont stage of Plasmodium spp. showing merozoites (1000x).
a) Leishman stained peripheral smear of blood showing gametocyte stage of Plasmodium vivax showing condensed eosinophilic nuclear chromatin (1000x); b) Leishman stained peripheral smear of blood showing gametocyte stage of Plasmodium vivax showing condensed eosinophilic nuclear chromatin (1000x).
(d) Artefacts Resembling Acid fast bacilli:
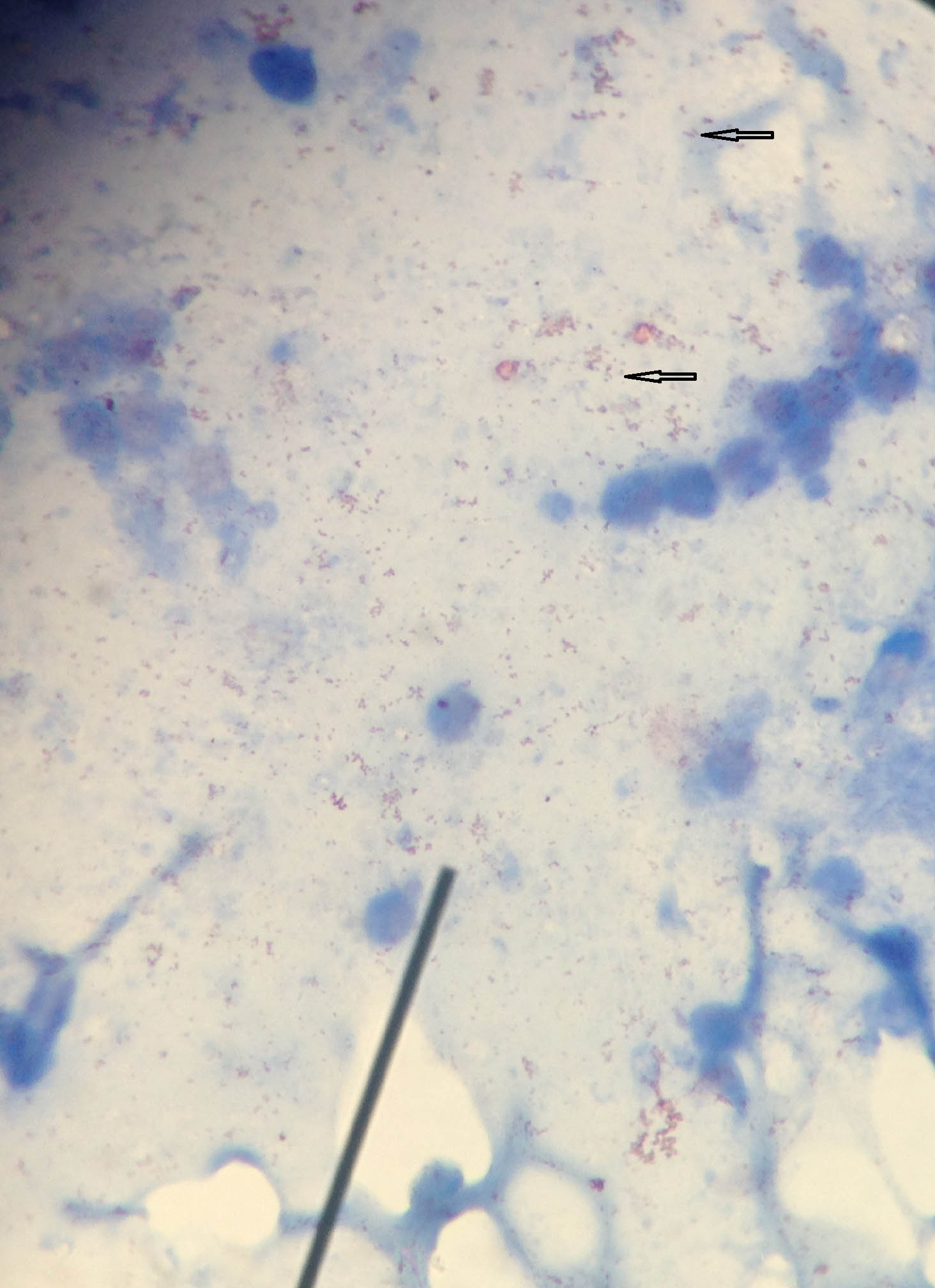
Case 1: Ziehl-Neelsen stained smear of sputum sample showing various non-uniform acid fast structures has been depicted in [Table/Fig-16]. These structures were confused with and therefore misidentified as acid fast bacilli.
Ziehl-Neelsen stained smear of sputum sample showing various non-uniform acid fast structures (1000x).
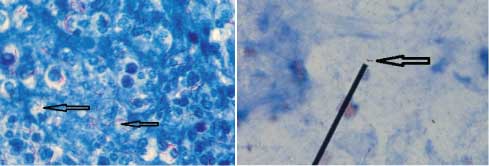
Case 2: Acid fast bacilli observed in sputum samples of a patient before and after starting treatment with anti-tubercular drugs have respectively been depicted in [Table/Fig-17a,b]. These bacilli appeared long and beaded prior to initiation of therapy but short & fragmented after treatment was started.
a) Ziehl-Neelsen stained smear of sputum of a patient showing acid fast bacilli before starting treatment with anti-tubercular drugs (1000x); b) Ziehl-Neelsen stained smear of sputum of a patient showing acid fast bacilli after starting treatment with anti-tubercular drugs (1000x).
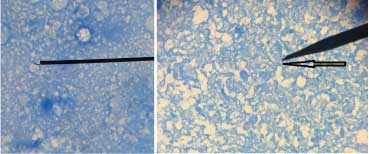
Case 3: Short acid fast bacilli observed in lymph node aspirate and brain abscess pus obtained from two patients have been shown in [Table/Fig-18a,b] respectively. The diagnosis of tuberculosis was confirmed by CB-NAAT (Cartridge based nucleic acid amplification technique).
a) Ziehl-Neelsen stained smear of lymph node aspirate obtained from a patient showing short acid fast bacilli (1000x); b) Ziehl-Neelsen stained smear of brain abscess pus obtained from a patient showing short acid fast bacilli (1000x).
(e) Artefacts Resembling Budding yeast cells:
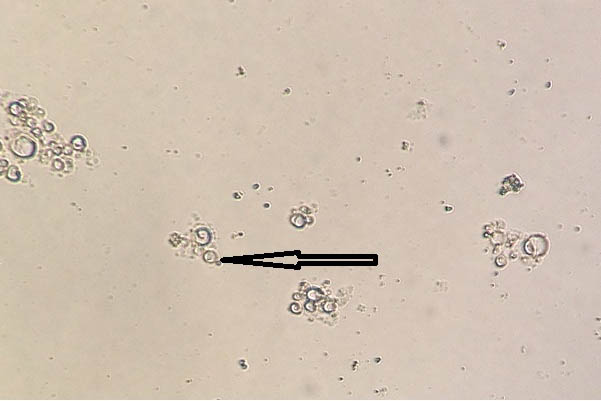
Case 1: KOH mount of spinal cord tissue sample obtained from a patient showing structures morphologically resembling budding yeast cells has been shown in [Table/Fig-19]. However, both aerobic bacterial and fungal culture of this sample were sterile thereby negating our preliminary microscopic findings.
KOH mount of spinal cord tissue sample showing structures morphologically resembling budding yeast cells (400x).
KOH mount showing yeast cells with germ tube formation have been depicted in [Table/Fig-20].
KOH mount showing yeast cells with germ tube formation (400x).
(f) Artefacts Resembling Hooklets Of Echinococcus Granulosus:
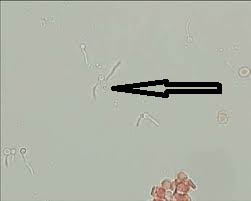
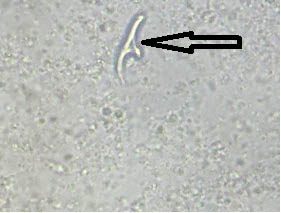
Case 1: Translucent structures morphologically resembling hooklets of Echinococcus granulosus observed in wet mount of fluid aspirated from hepatic cyst of a patient have been depicted in [Table/Fig-21a,b] respectively. Owing to absence of structures typically resembling hooklets of E. granulosus, this sample was reported as negative.
a) Translucent structures morphologically resembling hooklets of Echinococcus granulosus observed in wet mount of fluid aspirated from hepatic cyst of a patient (400x); b) Translucent structures morphologically resembling hooklets of Echinococcus granulosus observed in wet mount of fluid aspirated from hepatic cyst of a patient (400x).

Case 2: Charcot-Leyden crystals were also often confused with and wrongly identified as hooklets of E. granulosus. These are rhomboid shaped translucent structures as depicted in [Table/Fig-22].
Wet mount fluid aspirated from hepatic cyst of a patient showing Charcot-Leyden crystals (400x).
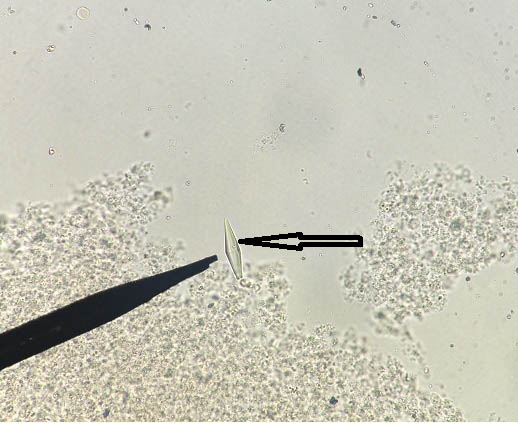
Typical ‘Y’ shaped refractile hooklet of E. granulosus has been shown in [Table/Fig-23].
Wet mount fluid aspirated from hepatic cyst of a patient showing typical ‘Y’ shaped refractile hooklet of E. granulosus (400x).
Discussion
The word artefact is derived from a Latin word “arsfactum” which literally means art (“ars”) + made (“factum”). It signifies any artificial feature or structure accidentally introduced into the specimen that is under study [1]. A microscopic artefact can lead to misdiagnosis that in turn may hamper appropriate treatment of the patient [2].
Artefactual changes may make familiar and common organisms exhibit an unfamiliar morphology, causing diagnostic difficulties and uncertainities. Dead capsulated bacteria may swell, mimicking the size and/or shape of fungal yeasts or some parasites. Antibiotic treatment may cause bacteria to replicate without complete division, giving the appearance of different bacteria, or even fungi [3]. Sutton BJ et al., reported two cases in which direct smears of body fluids containing filamentous bacteria were misinterpreted as fungal hyphae, with initiation of antifungal medication in one patient [4]. In a study conducted by Sangoi AR et al., it was found that only 79% of fungi were correctly identified based on morphologic features alone [5]. Fibrin, collagen and exogenous fibres may also mimic fungal hyphae [6]. Similar artefacts resembling fungal hyphae microscopically were observed in the present study as depicted in [Table/Fig-1,3,4 and 5b].
Many artefacts may be mistaken for parasites by inexperienced laboratory personnel. These include pollen or plant cells, white & red blood cells respectively, yeasts, hair, starch granules, macrophages and mucosal epithelium. Charcot-Leyden crystals are crystallized structures of varying sizes which originate from eosinophils and may sometimes confuse inexperienced microscopists. These are found in faeces, sputum & body tissues of patients with allergies and helminthic infestations [7]. In the present study also, charcot-leyden crystals were misidentified as hooklets of Echinococcus granulosus as shown in [Table/Fig-22]. Bits of cotton fibre, lint and other components of dust can mimic microfilariae in wet mounts or stained smears. However, an important feature that distinguishes these artefacts from microfilariae is absence of internal nuclei [8]. Similar observations were made in the present study as depicted in [Table/Fig-8].
Defective blood film preparation may lead to artefacts that are often interpreted as malaria parasites. In thin films, platelet clumps lying on top of or beside red blood cells especially when associated with a small fragment of blue stained material might cause confusion. Howell Jolly bodies, which are basophilic nuclear remnants or clusters of DNA in circulating erythrocytes, may also look like parasites when associated with blue stain deposits. The most common forms of artefact seen in thick films are those in which Howell Jolly bodies are seen associated with the ‘clouds’ of reticular material derived from lysed immature or degenerated red blood cells. In a poorly made film, there may be a chance association of some chromatoid debris with bluish stain deposits and these artefacts may be particularly realistic if there is also some granular, pigment-like material present as well. Bacterial contamination of either the blood film or stain may lead to confusion with small rings of Plasmodium falciparum [9]. We came across many such microscopic structures during the course of this study which were wrongly identified as different developmental stages of malaria parasites as depicted in [Table/Fig-10,11 and 12b] respectively.
In Ziehl-Neelsen stained smears of clinical samples using carbol fuchsin as primary stain, mycobacteria typically appear as red beaded or banded rods (1–10 μm long and 0.2–0.6 μm wide). However, these bacteria can sometimes appear as coccoid or filamentous structures as a result of which the underlying aetiology may not be correctly elucidated [10]. The morphological variants of acid fast bacilli shown in [Table/Fig-17a,b] might lead us to the conclusion that initiation of anti-tubercular treatment could result in alteration of microscopic appearance of these bacteria. However, it is important to know that routine microscopy cannot differentiate between live and dead acid fast bacilli and hence cannot be used as a follow-up diagnostic test [11]. In the present study, morphological variants of acid fast bacilli in sputum samples obtained from a patient prior to and after receiving anti-tubercular therapy as shown in [Table/Fig-17a,b]. We also observed, short acid fast bacilli were observed in lymph node aspirate of a patient as depicted in [Table/Fig-18a,b] respectively.
Conclusion
The literature available on this topic is very less so it is absolutely essential to highlight the issue of erroneous microscopic diagnosis of various infective syndromes. Through this study, we have attempted to share our experience, which will probably result in better patient management especially in poor resource settings.
Key-Message
We have attempted to share our experience about microscopic artefacts, which will probably result in better patient management especially in resource poor settings.
[1]. Jadhav KB, Gupta N, Ahmed MB, Maltese cross: Starch artefact in oral cytology, divulged through polarized microscopyJ Cytol 2010 27:40-41.10.4103/0970-9371.6669821042536 [Google Scholar] [CrossRef] [PubMed]
[2]. Kardam P, Jain K, Mehendiratta M, Mathias Y, Kaleidoscope of oral artefacts: A vivid picture through light and polarizing microscopeIndian J Pathol Microbiol 2016 59:31-34. [Google Scholar]
[3]. Boue J, Kahwash BM, Kirby S, Kahwash SB, The morphologic identification of common organisms that may look alike in the general pathology practice: a brief reviewIbnosina J Med BS 2014 6(5):227-34.10.4103/1947-489X.210391 [Google Scholar] [CrossRef]
[4]. Sutton BJ, Parsons AC, Palavecino EL, Filamentous bacteria masquerading as fungi: a diagnostic pitfall in direct smear interpretation with report of two casesJ Clin Pathol 2011 64(10):927-29.10.1136/jcp.2011.08928421415055 [Google Scholar] [CrossRef] [PubMed]
[5]. Sangoi AR, Rogers WM, Longacre TA, Montoya JG, Baron EJ, Banaei N, Challenges and pitfalls of morphologic identification of fungal infections in histologic and cytologic specimens: a ten-year retrospective review at a single institutionAm J Clin Pathol 2009 131(3):364-75.10.1309/AJCP99OOOZSNISCZ19228642 [Google Scholar] [CrossRef] [PubMed]
[6]. Almarzooqi S, Leber A, Kahwash S, Artefacts and organism mimickers in pathology: case examples and review of literatureAdv Anat Pathol 2010 17(4):277-81.10.1097/PAP.0b013e3181e4ab9320574173 [Google Scholar] [CrossRef] [PubMed]
[7]. Ridley JW, Parasitology for Medical and Clinical Laboratory Professionals [Internet]Delmar Cengage Learning USA 2012 [cited 15 October 2017].Available from: https://www.google.co.in/url?sa=t&rct=j&q=&esrc=s&source=web&cd=13&cad=rja&uact=8&ved=0ahUKEwjj1PCu0fLWAhXKQI8KHUy8Dcw4ChAWCCwwAg&url=http%3A%2F%2Ffile.zums.ac.ir%2Febook%2F435Parasitology%2520for%2520Medical%2520and%2520Clinical%2520Laboratory%2520Professionals-John%2520W.%2520Ridley-1435448162-Delm.pdf&usg=AOvVaw37In6JJNY_OBKnQ0PdNGUj [Google Scholar]
[8]. Garcia LS, Diagnostic Medical Parasitology [Internet]ASM press USA 2006 [cited 22 September 2017]. Available from: https://books.google.co.in/books?id=TQysBAAAQBAJ&pg=PT1945&lpg=PT1945&dq=artefacts+mimicking+microfilaria&source=bl&ots=sBYrbj7cEP&sig=CCHdcNcR32tDmVYx25XU7LEF48&hl=en&sa=X&ved=0ahUKEwiI-4urgMHWAhXF6Y8KHYx9C8IQ6AEIQDAH [Google Scholar]
[9]. Anon, (n.d.). Malaria Tutorial - Artefacts - Guy Cox. [online] Available at: http://guycox.com/malaria/artefact.htm [Accessed 1 May 2018] [Google Scholar]
[10]. Baron S, Medical Microbiology4th edition[Internet]. University of Texas Medical Branch at Galveston, Texas 1996 [cited 15 October2017]. Available from: https://www.google.co.in/url?sa=t&rct=j&q=&esrc=s&source=web&cd=7&cad=rja&uact=8&ved=0ahUKEwjezdy42PLWAhVBQI8KHcEcBckQFggMAY&url=https%3A%2F%2Fwww.ncbi.nlm.nih.gov%2Fbooks%2FNBK7812%2F&usg=AOvVaw3eHJAJ6N-Zbn2Ep6fR33FO [Google Scholar]
[11]. Oommen S, Banaji N, Laboratory diagnosis of tuberculosis: Advances in technology and drug susceptibility testingIndian J Med Microbiol 2017 35:323-31.10.4103/ijmm.IJMM_16_20429063875 [Google Scholar] [CrossRef] [PubMed]