Introduction
While de novo Ameloblastic Carcinomas (ACs) are easily diagnosed, it is the benign Ameloblastoma (AM) showing areas of malignant transformation which is a diagnostic challenge. SOX2, a transcription factor and Epidermal Growth Factor Receptor (EGFR) are oncogene on virtue of being embryogenic including odontogenic and adult stem cell regulator. Both are aberrantly expressed and amplified in several epithelial human cancers and have been used as immunohistochemical markers.
Aim
To determine the expression of SOX2 and EGFR in AM, Odontoameloblastoma (OA) and AC in order to assess efficacy of the markers in differentiating between these tumours.
Materials and Methods
This retrospective study was conducted to determine the immunohistochemical expression of SOX2 and EGFR on microscopic sections of AM (n=11), OA (n=2) and AC (n=6) retrieved from archives of the Department of Oral Pathology, Vydehi Institute of Dental Sciences, Bengaluru, India, from the period of January 2010 to December 2015. The data obtained were analysed using statistical software IBM SPSS version 21.0.
Results
EGFR expression was noted in all cases of AM, OA and AC. Eight cases (72.72%) of AM showed SOX2 negative expression. Five cases (83%) of AC showed SOX2 positive expression (p≥0.05). Both the cases of OA demonstrated SOX2 positivity. Two cases (50%) of recurrent AMs (n=4) showed SOX2 overexpression.
Conclusion
While SOX2 has negative expression in AM, its positivity in OA and AC reiterates, its role in presence of cell lineage of tooth development and as an adjunct marker to highlight suspicious tumour aggregates respectively. SOX2 overexpression in recurrent cases of AM can be used to follow-up the patient. Strong EGFR overexpression indicates possibility of anti-EGFR treatment modality for both AM and AC.
Biomarkers, Immunostaining, Odontogenic carcinoma, Odontogenic tumour, Oncogene
Introduction
Ameloblastoma is an uncommon, benign, but locally invading odontogenic neoplasm of the jaw. OA is a benign odontogenic jaw tumour which shows microscopic features of AM with mature or immature dental tissues. AC is a rare malignant odontogenic epithelial neoplasm which retains histologic features of ameloblastic differentiation with cytologic atypia, regardless of whether it has metastasised [1]. According to WHO classification (2005), AC is classified into two types, primary type occurs de novo and secondary type arises due to pre-existent benign AM undergoing malignant transformation [1,2].
Secondary type of AC are rare compared to de novo AC [3], whereas in a literature review of 31 cases from 2005 to 2011, majority of AC cases are seen to be arising from pre-existing AM and only 25% to be de novo cases [2]. In the latest WHO classification (2017), however, AC has been described as a single entity, since the authors found no justification in dividing a rare lesion. Similarly these authors believe that OA is a tumour arising from primitive ectoderm present in the odontome and discuss it as AM [4]. Irrespective of classification, AC show overt cytologic atypia with or without microscopic features of classical AM [3,5,6]. Some of the authors have advocated the use of SOX2 as a marker in routine practice to avoid overlooking such areas [3,7].
SOX2 is a member of the SOX family of transcription factors that share homology with the High Mobility Group (HMG) domain of the Sex determining Region Y (SRY) protein. It is a critical regulator of embryogenesis and is necessary for cellular reprogramming. SOX2 is responsible for development of ectoderm, its derivatives like odontogenic epithelium, dental lamina which are the precursors of tooth development. Thus, it participates in the maintenance of all types of epithelial proliferative pool of tooth development [7,8]. As it modulates embryonic stem cell self-renewal and differentiation, it is considered as an oncogene in many epithelial tumours. Its expression has been linked to more aggressive clinical course and poorer outcomes [8-10].
EGFR is a receptor tyrosine kinase with four predominant binding sites and multiple tyrosine residues for auto phosphorylation. Upon binding of the ligand, EGFR is activated, which initiates several downstream signaling pathways, responsible for cell lineage determination, cellular proliferation, cell homeostasis, organ morphogenesis, cellular motility and cell survival [11]. EGFR gene when disrupted, produces receptor proteins which results in abnormal cell growth or tumourigenesis giving EGFR oncogene status. Epithelial malignancies involving various organs like colon, ovaries, kidneys, breast along with head and neck squamous cell carcinomas show overexpression of EGFR with correlation to clinical course, treatment modality and outcome [12].
SOX2 and EGFR mediated signals both are required for self-renewal of adult stem cells. It has been observed that cancer stem cells have biological features similar to stem cells, therefore they may share the same regulatory signaling pathways [8,9,12].
SOX2 and EGFR are aberrantly expressed and amplified in several human cancers and have been used as markers in ovarian cancer, urothelial carcinoma, breast tumours, nasopharyngeal carcinoma and oral squamous cell carcinoma [9]. SOX2 directly upregulates EGFR expression whereas EGFR-mediated signaling promotes SOX2 production. The role of this feedback loop has been elucidated in lung cancer and glioblastoma [13]. This study is an attempt to shed some light on SOX2 EGFR expression in AM, OA and AC, and to assess their efficacy as marker in differentiating between these tumours.
Materials and Methods
This was a retrospective study conducted on the histologically diagnosed cases of AM obtained from the archives of the Department of Oral Pathology, Vydehi Institute of Dental sciences, from January 2010 to December 2015. On reviewing the microscopic slides of 19 cases reported previously as AM, six cases had areas fulfilling criteria of AC and two cases of OA. Only those cases were selected which had complete clinical history.
This study was approved by Institutional Ethical Board and involved the use of formalin fixed paraffin embedded tissues. Two sections of 4 μm each were obtained and transferred on to poly-L-lysine coated slides for immunostaining with SOX2 and EGFR. Sections were kept in incubator at a temperature range of 37-40°C overnight. The slides were deparafinised at 65-70°C for 15 minutes and then transferred to two changes of xylene for duration of 10 minutes each and then two changes of alcohol for 5 minutes each. Antigen retrieval was carried out by immersing slides in tris ethylene diamine tetra acetic acid buffer in a pressure cooker under pressure of 14 pounds per sq. inch for 5-10 minutes. The sections were then subjected to peroxide block for 15 minutes to block endogenous peroxide activity and incubated with the primary antibodies for 45 minutes at room temperature in a humid chamber. The primary antibodies used were anti SOX2 and anti EGFR monoclonal rabbit antibodies, procured from Path n Situ Biotechnologies Pvt. Ltd. The sections were then subjected to treatment with target binder and HRP polymer for 12 minutes each, followed by application of DAB chromogen for 5 minutes. In between the steps, the sections were washed using the immunowash buffer. The slides were counter stained with Harris haematoxylin, then washed in running tap water, dehydrated in ascending grades of alcohol, subjected to treatment with xylene for clearing, mounted with glass coverslip and DPX. Glioma and skin sections were used as positive controls of SOX2 and EGFR respectively. As a negative control, sections were stained without addition of a primary antibody.
Immunohistochemical analysis: Immunostains were interpreted independently by two pathologists. The scores were recorded based on the percentage of staining and intensity of staining in the cells of interest. SOX2 staining is localised to the nucleus. Percentage of staining was graded 1-4, that is 0-25%, 26-50%, 51-75% and 76-100%. Intensity was recorded from 0-3 representing negative, minimal, intermediate and strong respectively. The multiplied values give final score, in which <6 is considered negative [Table/Fig-1].
Descriptive analysis including scores for the percentage of SOX2 staining and intensity of all cases.
| Case No. | SOX2 | Final Score |
|---|
| % of Cell Staining | Staining Intensity |
|---|
| 0-25%Score-1 | 26-50%Score-2 | 51-75%Score-3 | 76-100%Score-4 | Score 0(no staining) | Score 1(weak staining) | Score 2(moderate staining) | Score 3(strong staining) | % of cell staining multiplied by staining intensity |
|---|
| 1 | * | | | | | * | | | 1 |
| 2 | | * | | | | | * | | 4 |
| 3 | * | | | | * | | | | 0 |
| 4 | | | * | | | | * | | 6 |
| 5 | * | | | | * | | | | 0 |
| 6 | * | | | | * | | | | 0 |
| 7 | | | * | | | | | * | 9 |
| 8 | * | | | | * | | | | 0 |
| 9 | * | | | | | * | | | 1 |
| 10 | * | | | | * | | | | 0 |
| 11 | | | | * | | | | * | 12 |
| 12 | | | * | | | | * | | 6 |
| 13 | | * | | | | | | * | 6 |
| 14 | | | * | | | | | * | 9 |
| 15 | * | | | | * | | | | 0 |
| 16 | | * | | | | | | * | 6 |
| 17 | | * | | | | | | * | 6 |
| 18 | | | | * | | | | * | 12 |
| 19 | | * | | | | | | * | 6 |
Where, * represents positive cells
EGFR staining noted in odontogenic cells was membranous and cytoplasmic. The percentage of positively stained tumour cells was calculated by dividing the number of EGFR positive cells by total number of tumour cells in a given field. The final EGFR score was calculated by multiplying percentage of tumour cells stained positively by stain intensity, where stain intensity was graded from 0-2 representing no staining, weak staining and strong staining respectively [Table/Fig-2].
Descriptive analysis including scores for the percentage of EGFR staining and intensity of all cases.
| Case No. | EGFR | Final Score |
|---|
| % of Cell Staining | Staining Intensity |
|---|
| 0- 25% | 26-50% | 51-75% | 76-100% | Score 0 (no staining) | Score 1 (weak staining) | Score 2 (strong staining) | % of cell staining multiplied by staining intensity |
|---|
| 1 | - | - | - | * | - | - | * | 2 |
| 2 | - | - | - | * | - | - | * | 2 |
| 3 | - | - | - | * | - | - | * | 2 |
| 4 | - | - | - | * | - | - | * | 1.8 |
| 5 | - | - | - | * | - | - | * | 1.7 |
| 6 | - | - | - | * | - | - | * | 2 |
| 7 | - | - | - | * | - | - | * | 2 |
| 8 | - | - | - | * | - | - | * | 2 |
| 9 | - | - | - | * | - | - | * | 2 |
| 10 | - | - | - | * | - | - | * | 1.8 |
| 11 | - | - | - | * | - | - | * | 2 |
| 12 | - | - | - | * | - | - | * | 2 |
| 13 | - | - | - | * | - | - | * | 2 |
| 14 | - | - | - | * | - | - | * | 2 |
| 15 | - | - | - | * | - | - | * | 2 |
| 16 | - | - | - | * | - | - | * | 2 |
| 17 | - | - | - | * | - | - | * | 2 |
| 18 | - | - | - | * | - | - | * | 2 |
| 19 | - | - | - | * | - | - | * | 2 |
Where, * represents positive cells
Statistical Analysis
The statistical software IBM SPSS version 21.0 was used for the analysis of the data. Descriptive statistical analysis of the data has been done in present study. Results on continuous measurement are presented on mean±SD (Min-Max) and results on categorical measurement are presented in number (%). Significance level for the study was assessed at 5%. Mann-Whitney test has been used to find out the significance of SOX2 expression between AM and AC. Pearsons coefficient was used to find out correlation of SOX2 expression and clinical variables.
Results
The study group comprised 15 (79%) cases of male patients and 4 (21%) female patients with an age ranging from 17-56 years. Mean age of the patients were 33.42±12.17 years. The clinical and histological features of all cases are included in [Table/Fig-3]. The six cases fulfilling histological criteria of AC are described in [Table/Fig-4]. Mann-Whitney test demonstrated higher SOX2 score in AC compared to AM however, the difference between the two was not statistically significant (p>0.05) [Table/Fig-5]. Overall SOX2 staining was negative or scanty in 8 (72.72%) cases of AM whereas 5 (83%) cases of AC had score ≥6 [Table/Fig-6a,b]. While one AM with history of no recurrence showed strong SOX2 positivity, 50% of recurrent AM cases (2/4) showed strong SOX2 positivity [Table/Fig-7a,b]. Both the cases of OA showed positivity (score 6) [Table/Fig-8]. Pearsons coefficient showed correlation between duration of tumour and SOX2 expression. SOX2 overexpression was seen in cases with shorter clinical course, but was not statistically significant (p=0.14). All the specimens demonstrated EGFR-positive tumour cells. There was no difference in EGFR scores between AM, OA and AC [Table/Fig-9a,b].
Clinical and histologic details of the ameloblastoma and ameloblastic carcinoma cases.
| Case no. | Age/Gender | Duration | Site | Size(in cm) | Pathological type | Treatment Done | EGFR | SOX-2 | Recurrence |
|---|
| 1 | 33/M | 6 months | Left mandible | 9×5 | Acanthomatous | Segmental resection | 2 | 1 | - |
| 2 | 20/M | 2 months | Right mandible | 4×5 | Plexiform | Segmental resection | 2 | 4 | Dentigerous cyst to Ameloblastoma |
| 3 | 32/F | 8 months | Left mandible | 6×5 | Follicular(mixed) | Hemimandibulectomy | 2 | 0 | Present |
| 4 | 19/F | 4 months | Left mandible | 8×5 | Plexiform | Hemimandibulectomy | 2 | 6 | Present |
| 5 | 48/M | 6 months | Left mandible | 2×2 | Mixed Type | Segmental resection | 2 | 0 | - |
| 6 | 53/M | 12 months | Right mandible | 9×4 | Acanthomatous | Segmental resection | 2 | 0 | - |
| 7 | 23/M | 3 months | Left mandible | 4×3 | Plexiform | Incisional biopsy | 2 | 9 | - |
| 8 | 25/F | 1 years | Right mandible | 8×2 | Acanthomatous | Surgical excision | 2 | 0 | - |
| 9 | 27/F | 6 months | Left mandible | 4×3 | Plexiform(mixed) | Segmental resection | 2 | 1 | - |
| 10 | 36/M | 6 months | Left mandible | 8×8 | Acanthomatous | Segmental resection | 2 | 0 | - |
| 11 | 35/M | 5 months | Left maxilla | 5×3 | Mixed | Maxillectomy | 2 | 12 | Recurrence as 3 times |
| 12 | 17/M | 8 months | Right mandible | 2×3 | Odontoameloblastoma | Hemimandibulectomy | 2 | 6 | - |
| 13 | 47/M | 9 years | Right mandible | 13×11 | Odontoameloblastoma | Surgical excision | 2 | 6 | - |
| 14 | 22/M | 2 years | Left mandible | 8×5 | Ameloblastic carcinoma | Hemimandibulectomy | 2 | 9 | - |
| 15 | 50/M | 7 months | Anterior mandible | 10×6 | Ameloblastic carcinoma | Surgical excision | 2 | 0 | AM to AC |
| 16 | 27/M | 6 months | Left mandible | 11×7 | Ameloblastic carcinoma | Segmental resection | 2 | 6 | - |
| 17 | 56/M | 6 months | Left mandible | 5×5 | Ameloblastic carcinoma | Segmental resection | 2 | 6 | - |
| 18 | 28/M | 5 months | Anterior mandible | 6×5 | Ameloblastic carcinoma | Surgical excision | 2 | 12 | - |
| 19 | 37/M | 6 months | Left mandible | 4×3 | Ameloblastic carcinoma | Segmental resection | 2 | 6 | - |
M: Male; F: Female; AM: Ameloblastoma; AC: Ameloblastic carcinoma
Histopathological features of ameloblastic carcinoma cases.
| Case No. | h/p types | Basilar hyperplasia | Mitotic figures | Vesiculated nuclei | Clear cells, ghost cells | Keratin pearls | Peri neural invasion | Intravascular invasion | necrosis |
|---|
| Case 14 | Follicular | + | 2-4 HPF | + | | | | | + |
| Case 15 | Papillary pattern | + | 2-4 HPF | + | | | | | |
| Case 16 | Follicular | + | >8 HPF | + | + | + | | | + |
| Case 17 | Follicular | + | 4-6 HPF | + | | | | | + |
| Case 18 | Follicular | + | 4-6 HPF | + | + | + | + | + | + |
| Case 19 | Follicular | + | 4-6 HPF | + | | + | | | + |
HPF: High power field; +: present
SOX2 score according to Mann-Whitney test.
| Group | n | Mean | Standard Deviation | Z value | p-value |
|---|
| Group 1 | 13 | 3.46 | 4.03 | -1.45 | 0.14 |
| Group 2 | 6 | 6.50 | 3.99 |
Group 1: AM and OA: Group 2: AC
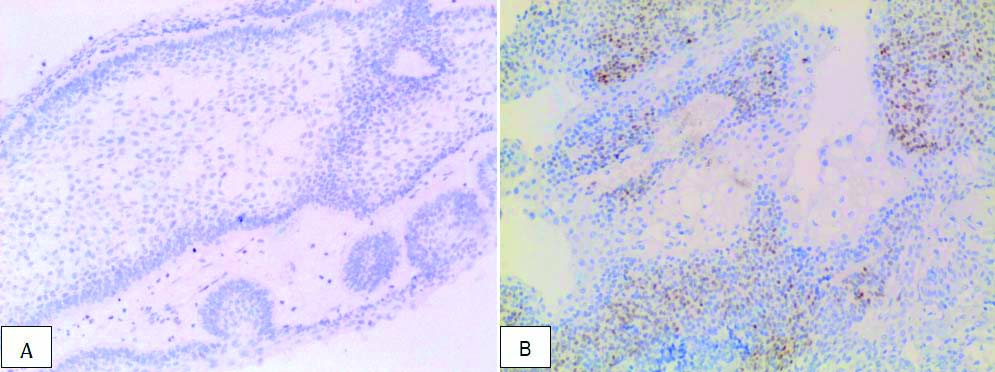
a) Negative SOX2 immunohistochemical expression in ameloblastoma in case no. 3 (20X magnification); b) Positive SOX2 expression in ameloblastic carcinoma (score 6) in case no. 17 (10X magnification).
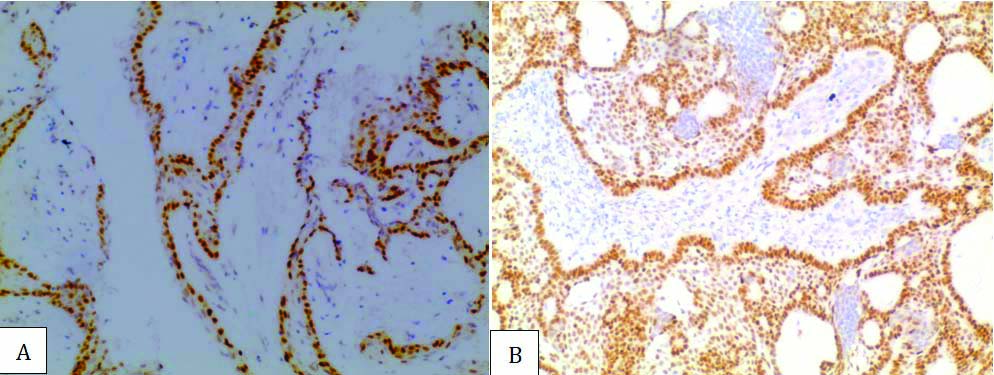
a) Positive SOX2 immunohistochemical expression (Score 9) in ameloblastoma with no recurrence in case no. 7 (20X magnification); b) Positive S0X2 immunohistochemical expression (Score 12) in third time recurrent ameloblastoma in case no. 11 (20X magnification).
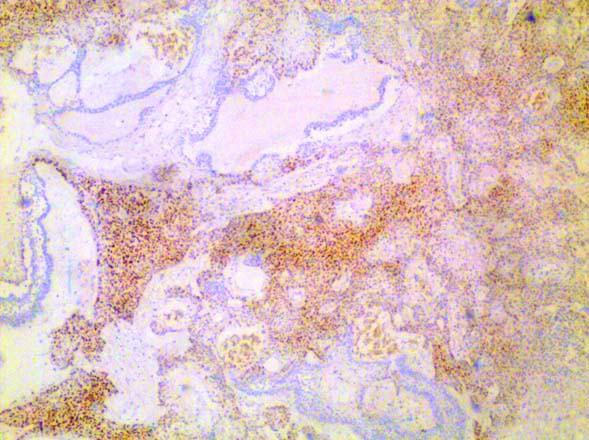
Positive SOX2 immunohistochemical expression in primitive cells in odontoameloblastoma in case no. 12 (10X magnification).
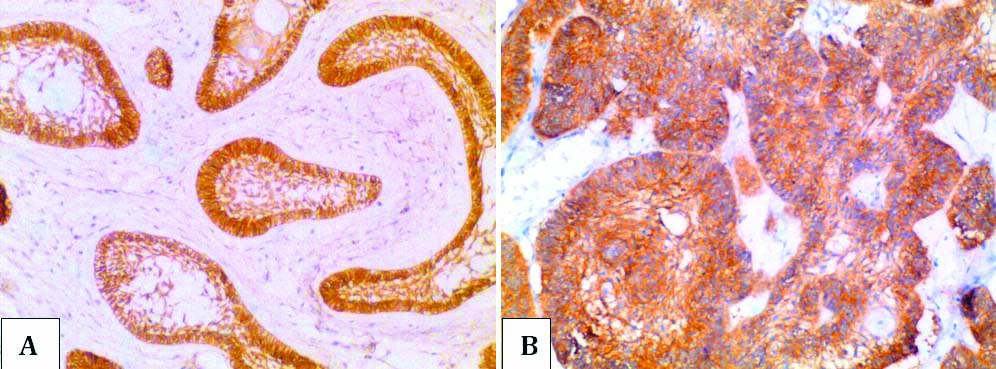
a) Positive EGFR expression (Score 2) in ameloblastoma in case no. 8 (20X magnification); b) Positive EGFR expression (Score 2) ameloblastic carcinoma in case no. 16 (20X magnification).
Discussion
Ameloblastic carcinoma is a malignant counterpart of AM with distinctive clinical, demographic and biologic characteristics but sometimes present with subtle histologic features [1]. The diagnosis of AC can be overlooked especially when it arises in a pre-existing AM. The histologic features of AM, such as peripheral palisading, reverse polarisation and/or stellate reticulum-like structures were present in all cases. The follicular, plexiform and/or trabecular growth patterns were found in present study. The suspicious areas of AC show follicles with basilar hyperplasia and the stellate reticulum-like structures replaced by the odontogenic cells. The cases of AC contained 2-10 mitotic figures per high-power field along with other features of malignancy such as hyperchromatism, nuclear pleomorphism, nucleus vesiculation and tumour necrosis. Infrequent features such as clear cells, ghost cells, keratin production, were also detected in some cases along with perineural invasion and vascular invasion. These features were consistent with histologic criteria for diagnosis of AC which is well described in the literature however, it can be missed out in secondary AC cases [5,6,14]. As a result, ancillary staining techniques like SOX2 have been recommended because of its high specificity and sensitivity to distinguish the AC from AM [7,15].
SOX2, a transcription factor is expressed in the stem cells during embryogenesis, tooth development as well as in various carcinomas of breast, colorectal, lung and squamous cell carcinomas [10]. In the present study, AM (8/11) cases were negative for SOX2, however recurrent cases of AM (2/4) showed strong SOX2 positivity. Cases of AC (5/6) showed SOX2 positivity in cytologically atypical areas. Lei Y et al., demonstrated strong nuclear positivity of SOX2 in tumour aggregates of AC compared to negative or scanty positivity in case of AM in their study with the reason being presence or absence of stem cell population respectively [7].
The role of SOX2 overexpression in recurrence and metastasis in head and neck squamous cell carcinoma has shown positive significance and has been linked to presence of cancer stem cells [10,16]. In a study done to compare SOX2 expression in AM to keratinizing cystic odontogenic tumour, latter with its high recurrence rate showed strong positivity which authors suggested might be because of stem cells [17]. Recurrence of AM has been linked to presence of SOX2 expressing stem cells [8]. This might explain the strong SOX2 overexpression in two cases of recurrent AM in the present study. In addition, one of the cases of AM had developed from dentigerous cyst, which showed moderate SOX2 positivity. According to Lei Y et al., caution to be taken when evaluating lesions arising from surface oral epithelium or from the dentigerous cyst, as basal cells of stratified squamous epithelium show SOX2 positive due to its role in epithelial homeostasis [7,10].
OA, a type of odontogenic tumour shows microscopic features of AM and various tissues of tooth in different stages of development [1]. The present study included two cases of OA, which showed SOX2 positivity of focal aggregates of tumour cells that lacked ameloblastic differentiation and exhibited moderate cytoplasmic or nuclear pleomorphism. This might be explained by fact that SOX2 shows positivity in epithelial cell lineage of tooth development [8].
EGFR is essential for normal physiological processes including odontogenesis. Disruption of receptor pathway leads to various cancers including those arising from remnants of odontogenic epithelium. EGFR overexpression has been demonstrated in AM [18,19]. It has been suggested that the combined cytoplasmic and membranous staining pattern of EGFR is indicative of proliferative potential of ameloblastomatous tumour islands similar to basal layer of oral epithelium and squamous cell carcinoma [18]. In the present study, all cases of AM, OA and AC exhibited similar intense EGFR immunoexpression. This result is similar to study by Abdel–Aziz A et al., who tried to elucidate the relationship between expression of EGFR, CD10 and Ki-67 labelling index and AM recurrence using clinical and pathological data and they could see no relation to recurrence [20].
It has been proven that SOX2 and EGFR have positive effect on each other in maintaining homeostasis of normal lung tissue, repair of damaged lung tissue by proliferating the stem cells and their overexpression in lung cancer [13]. However in the present study; no correlation between the expression of SOX2 and EGFR was elucidated in AM, OA and AC.
Limitation
However, in the small sample of AC studied, no clinicopathological correlation between marker expression, tumour progression and prognosis were discernable. These factors need to be explored within a larger study sample to elucidate the diagnostic and prognostic value of these markers.
Scope for future studies: Follow-up studies can be planned to see if recurrent AM cases with high SOX2 immunohistochemical expression do progress to AC and to validate, whether treatment planning of resection/ enucleation can depend on SOX2 immunohistochemical expression. Studies can be designed to investigate, if areas with high SOX2 immunohistochemical expression (as in OA) are suggestive of merely inductive changes/potential or they signal true malignancies. Also, it can be used for clarity of treatment planning in ambiguous cases, especially in differentiation of keratinizing cystic odontogenic tumour from unicystic AM.
Conclusion
SOX2 positivity in OA reiterates its role in cell lineage of tooth development needed for its diverse histopathological features. SOX2 immunohistochemical staining could serve as a useful marker to highlight unsure areas in AM. It was also positive for recurrent cases of AM and can be used to monitor the patients. AC shows a significant expression of SOX2 compared with AM, suggesting that differential expression of this marker could be used to support the diagnosis of AC. There was no correlation between EGFR and SOX2 staining pattern in AC and AM. However, strong positivity for EGFR may render both AC and AM as candidates for the new targeted anti-EGFR treatment modalities.
Where, * represents positive cells
Where, * represents positive cells
M: Male; F: Female; AM: Ameloblastoma; AC: Ameloblastic carcinoma
HPF: High power field; +: present
Group 1: AM and OA: Group 2: AC
[1]. Barnes L, Eveson JW, Reichart P, Sidransky D, World HealthOrganization Classification of Tumours Pathology and Genetics of Head and Neck Tumours 2005 LyonIARC Press:286-95. [Google Scholar]
[2]. Casaroto AR, Toledo GL, Filho JL, Soares CT, Capelari MM, Lara VS, Ameloblastic carcinoma, primary type: case report, immunohistochemical analysis and literature reviewAnticancer Research 2012 32(4):1515-26. [Google Scholar]
[3]. Richardson MS, Muller S, Malignant odontogenic tumours: an update on selected tumoursHead and Neck Pathol 2014 8:411-20.10.1007/s12105-014-0584-y25409848 [Google Scholar] [CrossRef] [PubMed]
[4]. Wright JM, Vered M, Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Odontogenic and Maxillofacial Bone TumoursHead and Neck Pathol 2017 11(1):68-77.10.1007/s12105-017-0794-128247226 [Google Scholar] [CrossRef] [PubMed]
[5]. Slater LJ, Odontogenic malignanciesOral Maxillofacial Surg Clin North Am 2004 16:409-24.10.1016/j.coms.2004.03.00918088742 [Google Scholar] [CrossRef] [PubMed]
[6]. Hall JM, Weathers DR, Unni KK, Ameloblastic carcinoma: an analysis of 14 casesOral Surg Oral Med Oral Pathol Oral Radiol Endod 2007 103:799-807.10.1016/j.tripleo.2006.11.04817448710 [Google Scholar] [CrossRef] [PubMed]
[7]. Lei Y, Jaradat JM, Owosho A, Adebiyi KE, Lybrand KS, Neville BW, Evaluation of SOX2 as a potential marker for ameloblastic carcinomaOral Surg Oral Med Oral Pathol Oral Radiol 2014 117(5):608-16.10.1016/j.oooo.2014.01.01724603057 [Google Scholar] [CrossRef] [PubMed]
[8]. Juuri E, Isaksson S, Jussila M, Heikinheimo K, Thesleff I, Expression of the stem cell marker, SOX 2, in ameloblastoma and dental epitheliumEur J Oral Sci 2013 121:509-16.10.1111/eos.1209524148099 [Google Scholar] [CrossRef] [PubMed]
[9]. Weina K, Utikal J, SOX2 and cancer: Current research and its implications in the clinicClinical and Translational Medicine 2014 3:1910.1186/2001-1326-3-1925114775 [Google Scholar] [CrossRef] [PubMed]
[10]. Ren ZH, Zhang CP, Ji T, Expression of SOX 2 in oral squamous cell carcinoma and the association with lymph node metastasis (Review)Oncology Letters 2016 11:1973-79.10.3892/ol.2016.420726998109 [Google Scholar] [CrossRef] [PubMed]
[11]. Ferguson KM, A structure-based view of epidermal growth factor receptor regulationAnnu Rev Biophys 2008 37:353-73.10.1146/annurev.biophys.37.032807.12582918573086 [Google Scholar] [CrossRef] [PubMed]
[12]. Yarden Y, The EGFR family and its ligand in human cancer. signaling mechanisms and therapeutic opportunitiesEur J Cancer 2001 37(Suppl 4):S3-8.10.1016/S0959-8049(01)00230-1 [Google Scholar] [CrossRef]
[13]. Chou YT, Lee CC, Hsiao SH, Lin SE, Lin SC, Chung CH, The emerging role of SOX2 in cell proliferation and survival and its cross talk with oncogenic signaling in lung cancerStem Cells 2013 31:2607-19.10.1002/stem.151823940081 [Google Scholar] [CrossRef] [PubMed]
[14]. Yoon HJ, Hong SP, Lee JL, Lee SS, Hong SD, Ameloblastic carcinoma: an analysis of 6 cases with review of the literatureOral Surg Oral Med Oral Pathol Oral Radiol Endod 2009 108:904-13.10.1016/j.tripleo.2009.06.04519800270 [Google Scholar] [CrossRef] [PubMed]
[15]. Hunter KD, Speight PM, The diagnostic usefulness of immunohistochemistry for odontogenic lesionsHead and Neck Pathol 2014 8:392-99.10.1007/s12105-014-0582-025409846 [Google Scholar] [CrossRef] [PubMed]
[16]. Lee SH, Oh SY, Do SI, Lee HJ, Kang HJ, Rho YS, SOX2 regulates self-renewal and tumourigenicity of stem-like cells of head and neck squamous cell carcinomaBritish Journal of Cancer 2014 111:2122-30.10.1038/bjc.2014.52825321191 [Google Scholar] [CrossRef] [PubMed]
[17]. Bandyopadhyay A, Nishat R, Behura SS, Panda A, Ramachandra S, Mohiddin G, Cancer stem cell markers, SOX2 and OCT 4 in ameloblastoma and keratocystic odontogenic tumour: an immunohistochemical studyJ Int Oral Health 2017 9:28-32.10.4103/0976-7428.201087 [Google Scholar] [CrossRef]
[18]. Vered M, Shohat I, Buchner A, Epidermal growth factor receptor expression in ameloblastomaOral Oncology 2003 39:138-43.10.1016/S1368-8375(02)00034-9 [Google Scholar] [CrossRef]
[19]. Mohan BC, Angadi PV, Role of epidermal growth factor receptor in odontogenic epithelium and development of odontogenic lesionsReceptors Clin Invest 2015 2:e824 [Google Scholar]
[20]. Abdel-Aziz A, Amin MM, EGFR, CD10 and proliferation marker Ki67 expression in ameloblastoma: possible role in local recurrenceDiagnostic Pathology 2012 7:1410.1186/1746-1596-7-1422300665 [Google Scholar] [CrossRef] [PubMed]