MCB is a rare subtype of breast carcinoma with aggressive behaviour and poor prognosis. Sub classification of this variant as per WHO has been attempted in our study since it is important to identify fibromatosis like variant with better prognosis. Any carcinoma of breast with mesenchymal or squamous differentiation is classified as metaplastic carcinoma. Detailed studies of MCB are less since these tumours constitute only 0.6% of all invasive breast cancers [1]. Assessment of Immunohistochemical (IHC) expression is necessary for prognostication and to plan treatment options. Moreover in cases with nodal metastasis, histopathology shows that it is usually the epithelial component that metastasizes. So it becomes important to analyse various histological patterns and IHC expression. This study is being done to assess the incidence, histopathology and IHC patterns of MCB in a tertiary care hospital in Kerala over a span of six years.
Materials and Methods
This is a descriptive study done in a tertiary care centre. A total of 36 cases of MCB diagnosed in the Department of Pathology, during the period of six years from January 2011 to December 2016 were analysed. Age distribution, histopathological and IHC patterns were studied using the registers, histopathology slides and IHC slides in the department. The study was accepted by Institutional ethics committee (IRB 52/2016). All mastectomy, lumpectomies and biopsies with diagnosis of MCB received in the histopathology lab during the six year period from January 2011 to December 2016 were included in our study. Cases with inadequate material were excluded from the study.
Serial sections were taken in relevant cases and Haematoxylin and Eosin (H&E) slides were prepared. IHC studies were done in paraffin blocks of all newly diagnosed cases of metaplastic carcinoma and in all possible cases which were previously diagnosed. The hormone receptor status (Oestrogen and progesterone receptor) and overexpression of HER 2/neu was studied by appropriate antibodies and grading was done. The antibodies were applied after antigen retrieval and then counterstained using diamino benzidine. Grading was done as per the guidelines published by American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP). ER/PR was graded after counting 300 cells with positive external control. For assessment of ER and PR status nuclear positivity in more than 1% of the tumour cells are taken as positive. For HER 2/neu, those tumours that show strong circumferential membrane staining that is complete and intense in >10% of tumour cells is taken as positive (3+). However, there were no HER2Neu 2+ cases in our study.
Statistical Analysis
The age, gender distribution, morphological and IHC patterns were studied and data analysis was done using Statistical Package for Social Sciences (SPSS) programme version 16.0.
Results
A total of 36 cases of MCB were received in the histopathology lab of our Institution over a six year period from January 2011 to December 2016. The age distribution varied between 34 to 72 years with mean age 51.8 years and median age of 49 years. Female: Male ratio was 35:1 with 97.2% of cases in females and 2.8% in males. The left breast was affected in 61.1% (n=22) of cases. Tumour size varied from 1.5 to 15 cm with a median size of 4.5 cm. Majority of cases appeared as grey white growths with infiltrative margins. Cut surface was predominantly solid in 75.9% cases and cystic change was identified in 24.1% (n=7cases). Gross specimen of a case shows a grey white growth with cystic change and infiltrative margins [Table/Fig-1a].
1a: Gross specimen showing a grey white growth with cystic change and infiltrative margins. 1b: Microscopy showing squamous areas and malignant cells in sheets (10X). 1c: Chondroid differentiation with cells showing nuclear atypia. (10X) and inset showing tumour giant cells. 1d: Malignant spindle cells in sheets 10X and inset showing spindly cells with nuclear atypia (40X).
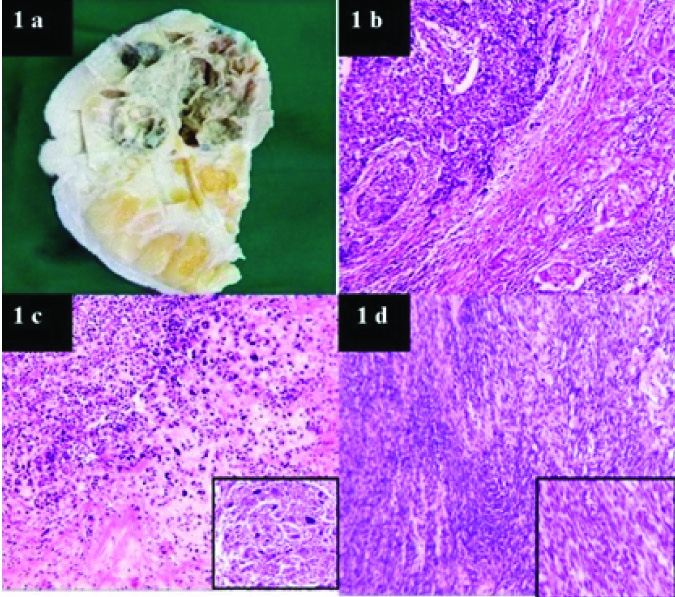
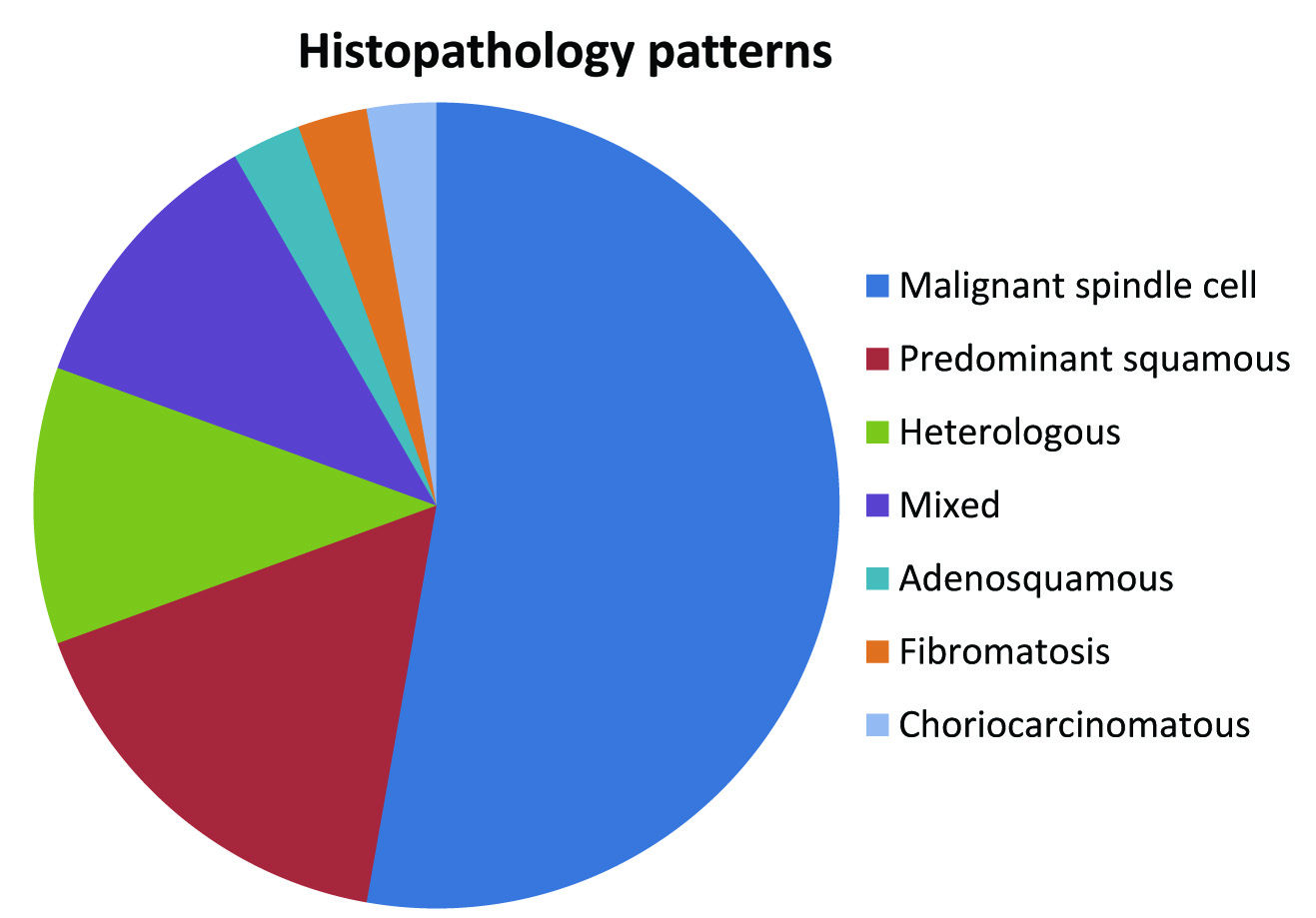
Histopathological findings indicate predominant squamous component in 17% cases [Table/Fig-1b]. Mixed pattern (different histopathologic patterns were seen in the same tumour) in 11%. Heterologous differentiation (chondroid, osseous) was seen in 11% cases [Table/Fig-1c], choriocarcinomatous component was identified in 3%. Fifty three percent cases showed malignant spindle cells in sheets and fascicles as the predominant histopathological pattern [Table/Fig-1d and (inset)]. Fibromatosis like MCB and adenosquamous type accounted for 3% each. Diagrammatic representation showing the percentage of the different histologic types is shown in [Table/Fig-2]. Squamous pearl formation was noted in cases with predominant squamous component [Table/Fig-3a,b].
Shows the percentage of the different histologic types.
3a: Microscopy showing adeno pattern and squamous pearl (10X). Inset showing ER positivity. (40X). 3b: Microscopy showing pure squamous pattern (10X) and Inset showing squamous pearl (40X). 3c: Showing metastasis to lymph node (10X) and Inset showing PR positivity (40X). 3d: Microscopy showing pleomorphic cells (40X) and Ki 67 positivity. {Inset (40X)}
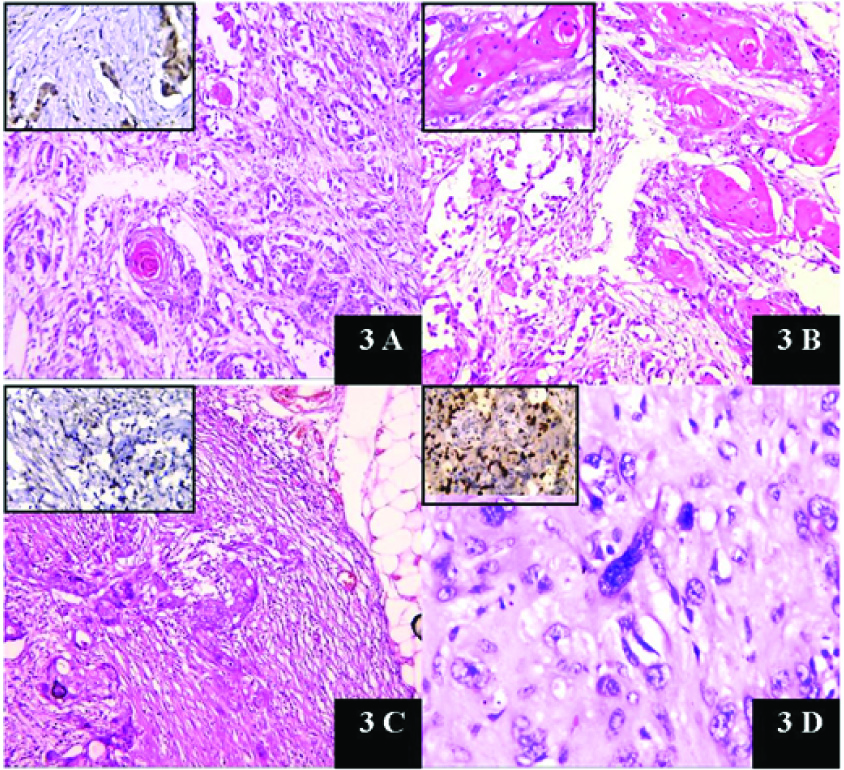
Some cases showed giant cell rich areas [Table/Fig-1c and (inset)] and some showed pleomorphic and bizarre cells [Table/Fig-3d].
Of the 7 cases diagnosed on trucut biopsies, four were lost to follow up and 3 cases were referred to other institutions. Hence tumour size, nodal status and lymphovascular invasion could not be assessed in these cases. However they were included in analysis of histopathological patterns.
Of the remaining 29 cases, lymphovascular invasion was found in 55.2% cases. Lymph node involvement was found in 41.4% cases (n=12), was absent in 51.7% and could not be assessed in two cases of wide excision specimens in which axillary dissection was deferred due to post operative complications. Malignant epithelial component was seen to metastasize to nodes [Table/Fig-3c]. Extra nodal spread was found in six cases. Four cases developed metastasis to lung, one developed brain metastasis and one case presented as thyroid metastasis. Immunohistochemistry work up was available in 26 cases only. Majority showed a Triple negative pattern 69.2% (n=18). Four cases (15.5%) cases were ER positive only, 3 ER & PR (11.5%) positive and 1 case showed positivity for PR only (3.8%). Ki 67 (done in 5 cases) showed increased proliferation (17-28%) [Table/Fig-3(inset)a,b,d]. All cases were done with positive controls [Table/Fig-4a-d]. [Table/Fig-5] shows the different IHC patterns in our study.
4a: Microscopy showing positive internal control ER positivity. (10X) black arrow shows normal duct. 4b: Microscopy showing positive external control for ER (40X). 4c: Microscopy showing positive external control for PR (40X). 4d: Microscopy showing positive external control for HER 2/neu (40X).
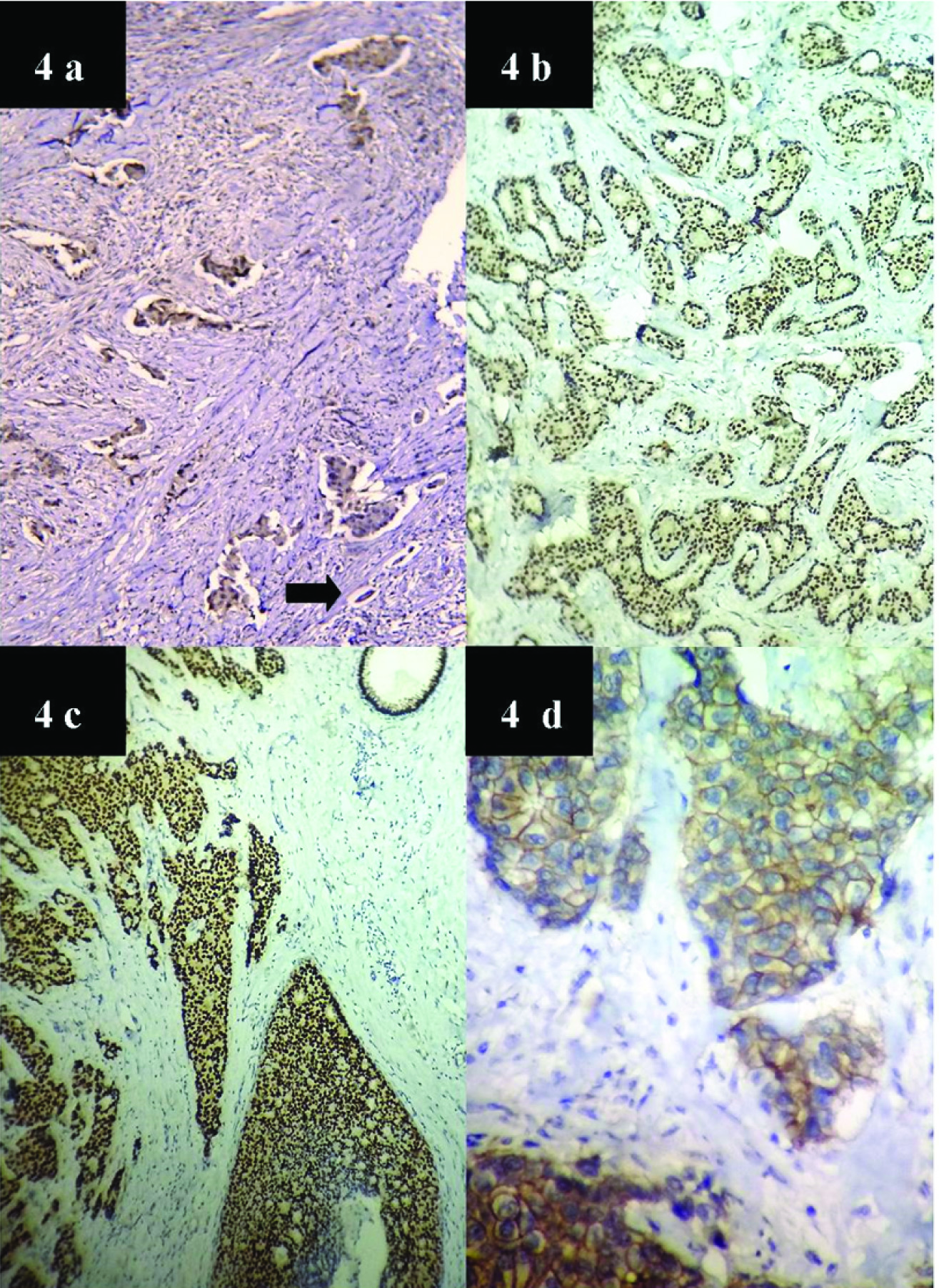
The IHC patterns obtained in our study.
| IHC pattern | Triple negative | ERPR Positive | ER positive | PR positive | Total cases |
|---|
| Number of cases | 18 | 3 | 4 | 1 | 26 cases |
| Percentage | 69.2 | 11.5 | 15.4 | 3.8 | 100%. |
Discussion
MCB is a rare heterogeneous group of primary breast malignancy accounting for only less than 1% of all invasive mammary carcinomas [1-3]. They are characterized by the co-existence of malignant epithelial and mesenchymal elements. The different subtypes included under descriptive classification of MCB by the WHO working group on breast tumours are based on the predominant cell type (spindle/squamous/mesenchymal) and the histological pattern [4]. Low grade adenosquamous and fibromatosis-like subtypes are reported to have a better prognosis [5]. Pathological classification of MCB and its differential diagnosis are challenging due to the diversity of the histological patterns, rarity of diagnosis and lack of consensus in classification of this group of tumours. Descriptive classification suggested by WHO has been effective in sub categorizing these tumours with diverse histopathological patterns and thus addressing these issues to an extent [4].
Of a total of 1405 cases of invasive breast carcinomas received in our institution during this study, 36 cases were metaplastic carcinoma thus accounting for 2.6% of invasive breast carcinomas. Age distribution varied between 34 to 72 years. Most studies have reported occurrence of MCBs in females over 50 years [1,2,6]. However in our study the median age was 49 years and about half of the cases were below 50 years. Ninety seven percent were females (n=35) and 2.8% (n=1) were males. Male: female ratio was 1:35. Majority of MCBs usually presents as a large size mass and are high-grade neoplasms. In our study, tumour size varied from 1.5 to 15cms with a median size of 4.5 cms. Comparisons with other studies are given in [Table/Fig-6]. Majority of cases appeared as solid grey white growths (75.9%) with infiltrative margins. However, 24.1% showed cystic degeneration. Most of the tumours with cystic change showed squamous component.
Comparison with other studies [10-14].
| Variables | Present study | Al Sayed AD et al., 2006 [10] | Boler DE et al., 2016[11] | Altaf FJ et al., 2014 [12] | Carter MR et al., 2006 [13] | Salimoqlu S et al., 2013 [14] |
|---|
| Author and year of publication |
|---|
| Number of cases | 36 | 19 | 7 | 7 | 29 | 11 |
| Median age | 4934-72 | 48range 14-58 | 5140-65 | 3623-63 | 6840-96 | 56 |
| Tumour sizeRange | 4.5cm(1.5-15) | 9cm(3-18) | 4cm(3.5-8.5) | 7cm(5-13) | 4cm(1.5-15) | 3.8(1.5-14) |
| Common histopathology pattern | MalignantSpindle cells(53%) | (53%)Mixed10cases | 57% (4/7) squamous component +3cases mixed | 71%(5/7) malignant spindle | MalignantSpindle 80% | 72%squamous8/11 |
| Common IHC pattern | 69.2%TN(18/26)ERPR +ve (11.5%)ER only +ve (15.5%)PR only +ve (3.8%)#Ki 67 index high | 82% TN (14/17)ERPR –ve (88%)15/171 /15 HER + | 86% TN(6/7) | 100%(7/7) TN | 100% TN | 55%TN(6/11)90%ERPR-Ve |
| Nodal status | 41.4%12/29 | 53% | 14%(1/7) | 57% (4/7) | 5%(1/20) | 45%(5/11) |
*TN: Triple negative; ER: Estrogen receptor; PR: Progesterone receptor; HER: Human epidermal growth factor.
(NB-#Ki 67 was done in 5 cases only and it was increased .Values ranged from 17-28%)
The different histopathological patterns encountered in our study were malignant spindle cells in sheets and fascicles as the predominant pattern in 53%, predominant squamous component in 17%, mixed type in 11%, heterologous differentiation was found (chondroid, osseous) in 11%, choriocarciomatous component (3%), fibromatosis like MCB and adenosquamous type accounted for 3% cases each.
MCBs usually arise de-novo, but there are rare reported cases that arose from pre-existing lesions such as complex sclerosing lesions and papillomas [7,8]. Two cases each of Ductal Carcinoma Insitu (DCIS) and fibrocystic disease and a single case of adenomyoepithelioma were detected as co-existent lesions in our study.
Most studies show MCB to have a triple negative phenotype [9]. That is, IHC studies show negativity for hormone receptors (estrogen and progesterone receptors) and low overexpression of HER 2/neu receptor [10]. In our study also IHC work up showed Triple Negative (TN) pattern in majority cases (69.2%). However ER and PR positive (11.5%) cases were also present but HER 2/neu overexpression was not detected in any of our cases. Similar IHC patterns have been reported in other studies but HER 2/neu overexpression has also been reported rarely [Table/Fig-6] [11]. Ki 67 was done in a few cases showed high proliferation index [Table/Fig-3d (inset)]. Positivity for IHC markers like Pan cytokeratin, vimentin, CK5/6, EGFR, p63 and SMA have been reported in certain studies [12].
Though lymph node involvement is less compared to that in invasive duct carcinoma, the chance of extra nodal metastasis especially to lungs has been reported in certain studies [13]. The presence of sarcomatous component was thought to be an explanation for decreased rate of nodal metastasis [6]. Lymph node metastasis was present in 41.4% cases (n=12) and majority of the metastasizing elements were malignant epithelial components especially squamous component. Huvos AG et al., has reported the association between axillary nodal metastasis and squamous sub type of MCB [15]. Sites of extranodal spread reported in literature include lung, liver, brain, chest wall, soft tissue, mediastinum, bone and kidney [16].
Assessment of histopathological component and IHC may help to predict the behaviour and prognosis of MCBs. Extensive sampling should be done in all cases which show squamous areas or spindle cells with atypia. Two of our cases which were diagnosed as invasive ductal carcinoma on trucut biopsy showed malignant mesenchymal elements in one and predominant squamous areas in the other in the mastectomy specimen. On reviewing the biopsy slides a small foci of atypical spindle cells were found in one while other showed squamoid cells with predominant areas of invasive ductal component in both. This would have resulted in an initial diagnosis of ductal carcinoma. IHC work up in both cases showed triple negative pattern with vimentin positivity (malignant mesenchymal component) and basal keratin positivity (in epithelial elements) thus favouring the diagnosis of MCB. Hence presence of any suspicious elements warrants adequate sampling and IHC work up.
All cases with nodal metastasis in our series showed malignant squamous or ductal elements. Tumour size, lymph node stage, Lymphovascular Invasion (LVI) and Ki67status are important in prognostication. In our study 55.2% cases showed LVI. Further studies are needed in deciding an appropriate management of these rare neoplasms. Different studies confirm that MCBs are aggressive tumours and patients generally have poorer outcome and rarely benefit from conventional chemotherapy or hormonal therapy [6,12,14,16].
Limitation
Limitations of our study include lack of follow up of patients in some cases, thus affecting assessments of metastasis and extra nodal spread which was done only in those cases with follow up. Also, detailed sampling and IHC studies could not be done on all cases; hence there might be bias in assessments of histopathology and IHC patterns.
Conclusion
The present study shows that though MCB accounts for a small percentage of all invasive breast carcinomas, it is an aggressive tumour with diverse histopathological patterns. Presence of atypical spindle cells in trucut biopsies should arouse suspicion of this aggressive variant of breast cancer and detailed sampling from mastectomy specimens may be necessary in certain cases to identify both malignant epithelial and mesenchymal elements. Sub categorization is important since a predominant malignant mesenchymal element confers the chance for extranodal spread. The percentage of MCBs, their histopathological and IHC characteristics are comparable with other studies.
*TN: Triple negative; ER: Estrogen receptor; PR: Progesterone receptor; HER: Human epidermal growth factor.
(NB-#Ki 67 was done in 5 cases only and it was increased .Values ranged from 17-28%)