Melioidosis: An Emerging Infectious Disease as a Cause of Parotid Abscess: A Case Report
Reshmi M. Nair1, Sonya Joy2, Dhanya Elappulli Krishnamurthy3
1 Senior Consultant, Department of Ear, Nose and Throat, Rajagiri Hospital, Aluva, Kerala, India.
2 Consultant Microbiologist and Infection Control officer, Aster Medcity, Kochi, Kerala, India.
3 Consultant, Department of Ear, Nose and Throat, Rajagiri Hospital, Aluva, Kerala, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Reshmi M. Nair, Senior Consultant, Department of Ear, Nose and Throat, Rajagiri Hospital, Chunangamveli, Aluva-683112, Kerala, India.
E-mail: reshmi.madhavan@rajagirihospital.com
Parotid abscess as a complication following parotitis is rare and it usually occurs due to salivary stasis, the most common organisms being isolated are Staphylococcus aureus and anaerobic bacteria. Burkholderia pseudomallei causing an isolated parotid abscess is rare in adults with very few reported cases. We present here a case of left sided parotid abscess in a diabetic female which on microbiologic evaluation turned out to be due to Burkholderia pseudomallei. She was successfully treated with a combination of injection ceftazidime six grams in three divided doses and cotrimoxazole (320mg +1600 mg) in two divided doses for initial two weeks followed by a maintenance phase with cotrimoxazole alone during the next 12 weeks.
Burkholderia pseudomallei, Diabetes mellitus, Parotitis, Salivary gland
Case Report
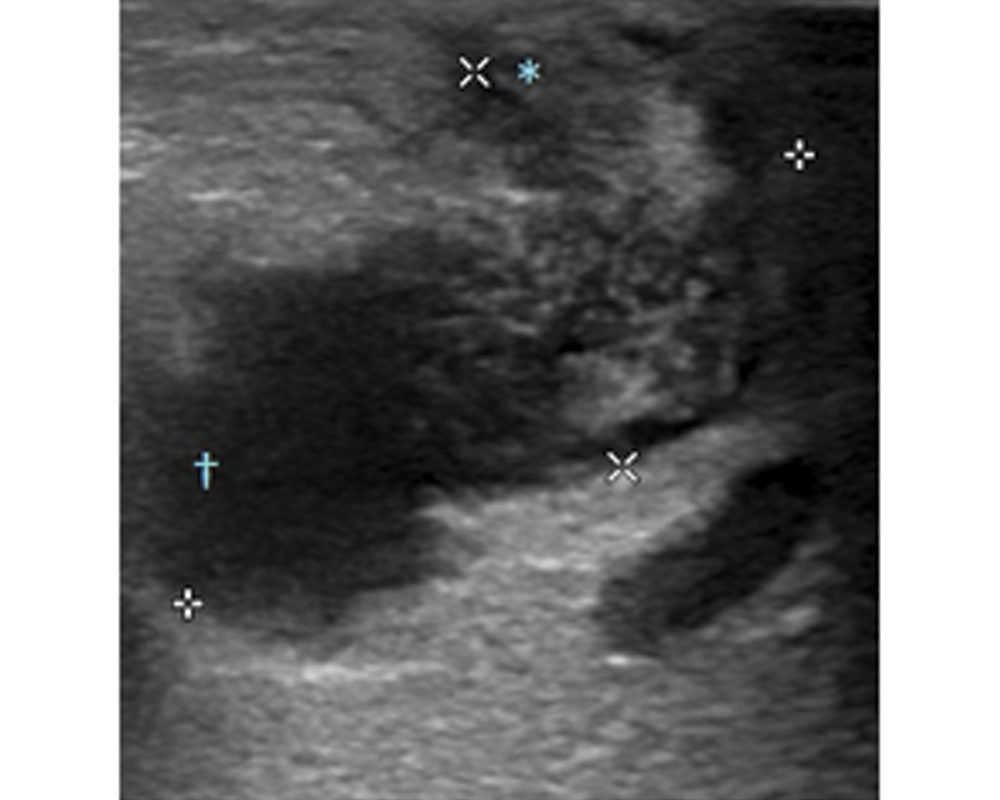
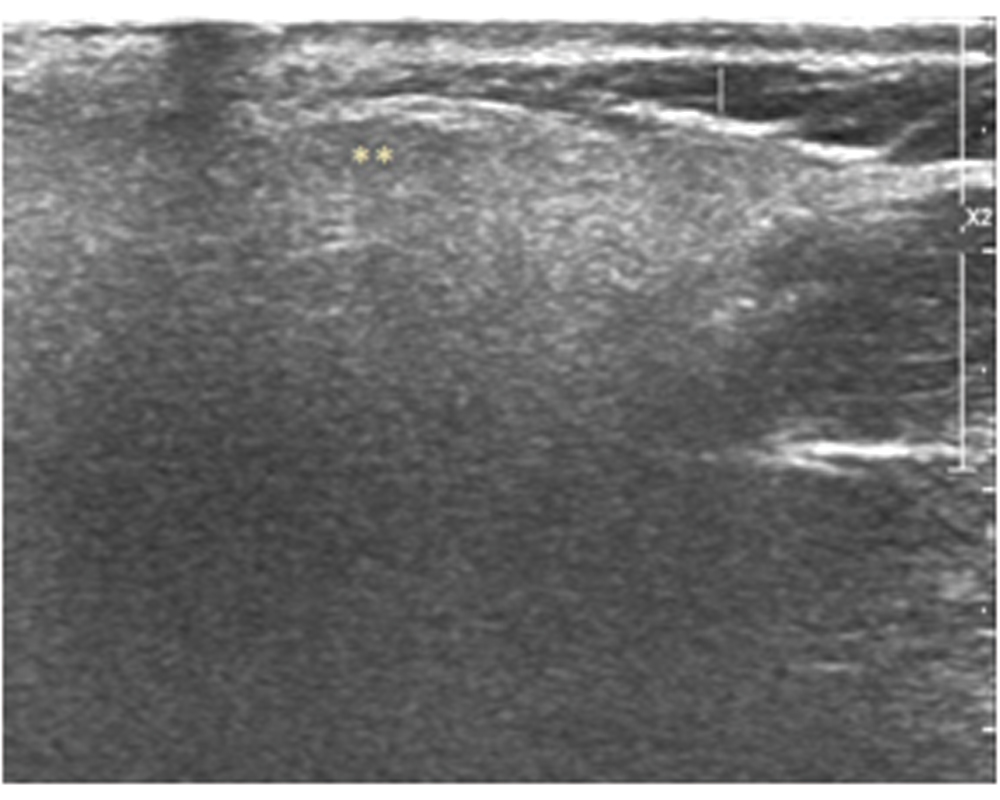
A 42-year-old diabetic female presented to our outpatient clinic with persistent parotid swelling on left side with recurrent episodes of pain over the swelling from two months. Even though there was partial resolution of pain with antibiotic and anti-inflammatory medication prescribed to her by the primary physician, the swelling did not regress in size. On clinical examination there was fullness and swelling at the left infraauricular and preauricular region. It was firm in consistency and tender on palpation with congested duct opening suggestive of parotitis on left side. There were no palpable cervical nodes and her facial nerve function was normal and symmetrical on both sides. The palpation of the stensons duct was done, no calculi could be felt. Her systemic examination was unremarkable. She was started on amoxycilln-potassium clavulanate 625 mg thrice daily dosage and paracetamol 650 mg for pain, but on follow up she had worsening of symptoms with increase in pain and size of swelling. An ultrasonogram was done which showed left intraparotid collection with subcutaneous extension [Table/Fig-1]. An ultrasound guided aspiration was done and sent for culture and sensitivity. A 1 cm incision was made at the fluctuant area which was marked under ultrasound guidance. The tract was explored with blunt instrument under local anaesthesia through the parotid fascia and pus drained. The cavity was packed with medicated gauze. A daily dilatation of the tract and dressing was done for two more days till there was no significant soakage of dressing. The pus culture by VITEK 2 automated identification system grew Burkholderiapseudomallei, sensitive to ceftazidime and cotrimoxazole. The patient was started on ceftazidime six gram per day intravenous in three divided doses and cotrimoxazole (320mg+1600mg) in two divided doses for the initial two weeks as intensive phase followed by maintenance phase with cotrimoxazole for 12 weeks. The glycaemic control was achieved with insulin aspart (Novorapid) eight units once daily along with oral hypoglycaemic medication metformin 1000 mg twice daily and sitagliptin 50 mg once daily. The patient had resolution of symptoms with decrease in size of swelling within two weeks of initiating the medication and is on regular follow up for last one year [Table/Fig-2].
Ultrasonogram of left parotid gland showing intraparotid collection with subcutaneous extension.
*subcutaneous extension of abscess /†collection within the gland 
 Symbols marking the extend of collection in two planes
Symbols marking the extend of collection in two planes
Follow up Ultrasonogram left parotid with normal parenchyma.
** normal parenchyma
Discussion
Melioidosis also called whitemore’s disease, is a zoonotic disease caused by Burkholderia pseudomallei which is an aerobic gram negative bacillus. This is a natural inhabitant of soil and water in tropics and subtropics mainly in South East Asia and Northern Australia [1]. There are cases being reported in India, mainly from the western coastal region of India, Tamil Nadu and Puducherry [2-4]. The disease has been increasingly reported over the last few years and there are few epidemiological studies too from the southern states of the Indian subcontinent [2, 3]. An early diagnosis is crucial to avoid morbidity and mortality as the disseminated disease presenting as septic shock has a fatality rate of around 20%-50% [1,2].
The disease is transmitted by inhalation, direct contact with skin lesion or contaminated water or food. The association between rainfall intensity and disease is well documented in endemic areas [3,5]. Our patient had the disease during the monsoon months and had exposure to soil as she used to do vegetable farming
The disease presents as localized in 50% of the cases or could be disseminated. The most common presentation is pneumonia occurring in around 30% of the cases [5]. In endemic areas, the parotid gland involvement is seen in around one third of paediatric patients affected with melioidosis, but it is rare in adults with very few reported cases [6-9].
The common risk factors are age more than 50, diabetes mellitus, chronic liver failure, chronic kidney disease, chronic lung disease, excessive alcohol consumption and immunosupression [5,10,11]. Diabetes mellitus is the single most common risk factor which is seen in around 23%-60% of the patients [11] and this patient too had diabetes mellitus.
An early diagnosis is crucial in melioidosis to avoid morbidity and mortality as the disseminated disease presenting as septic shock has a fatality rate of around 20%-50% [1,2]; however the diagnosis of melioidosis is challenging. Isolation of Burkholderia pseudomallei from clinical specimen culture is the gold standard for diagnosis [10,11]. Serological tests like ELISA for IgG and IgM antibodies and indirect haemagglutination tests are helpful in making a provisional diagnosis in the absence of isolation of the bacteria from the specimen [10]. Therapy includes prolonged medication to cure the disease and to prevent relapse. The antimicrobial therapy includes an initial intensive phase with parenteral medication followed by an eradication phase with oral medication. The recommended dose for deep seated tissue collection based on recommended guidelines by united states centre for disease control and prevention, 2010 [12] is ceftazidime 50 mg/kg upto two Gram intravenously eight hourly or Meropenem 25 mg/kg upto one Gram eight hourly in combination with trimethoprim and sulfamethoxazole for minimum of two weeks which may be extended to four weeks or longer depending on severity followed by trimethoprim+sulfamethoxazole -240 + 1,200 mg (for adult 40-60 kg) and 320 +1,600 mg orally (adult > 60 kg), 12-hourly for further three months. In present case, the patient received ceftazidime six gram in divided doses with trimethoprim and sulphamethoxazole 320+1600 mg for two weeks followed by cotrimoxazole alone in the same dose for three months [11,12].
Conclusion
The possibility of melioidosis needs to be considered in the differential diagnosis of recurrent parotitis especially in diabetics not responding to conventional treatment. A high index of suspicion, culture confirmation and a long term treatment regime gives excellent outcome in management of this emerging disease.
[1]. Currie BJ, Fisher DA, Howard DM, James NCB, David Lo, Selva Nayagam S, Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literatureClin Infect Dis 2000 31(4):981-86.10.1086/318116 [Google Scholar] [CrossRef]
[2]. Gopalakrishnan R, Sureshkumar D, Thirunarayan MA, Ramasubramanian V, Melioidosis: an emerging infection in IndiaJournal of the Association of Physicians of India 2013 61:612-14. [Google Scholar]
[3]. Vidyalakshmi K, Lipika S, Vishal S, Damodar S, Chakrapani M, Emerging clinico-epidemiological trends in melioidosis: analysis of 95 cases from western coastal IndiaInternational Journal of Infectious Diseases 2012 16:491-97.10.1016/j.ijid.2012.02.01222512851 [Google Scholar] [CrossRef] [PubMed]
[4]. Uttam U, Sagar C, Akshay D, Meena D, A new threat to children: MelioidosisPaediatric Infectious Disease 2014 6:135-38.10.1016/j.pid.2015.01.001 [Google Scholar] [CrossRef]
[5]. Currie BJ, Ward L, Cheng AC, The Epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year darwin prospective studyPLoS Negl Trop Dis 2010 4(11):e90010.1371/journal.pntd.000090021152057 [Google Scholar] [CrossRef] [PubMed]
[6]. Kamath MP, Bhojwani K, Chakrapani M, Vidyalakshmi K, Vishnuprasad KP, Meliodosis of salivary glands with coexisting diabetes: management of a difficult caseEar Nose Throat J 2014 93(1):e22-25. [Google Scholar]
[7]. Janakiraman R, Veeraraghavan B, Pranay G, John CM, Melioidosis of the parotid: the tip of the icebergHead Neck Surg 2008 139(5):731-32.10.1016/j.otohns.2008.07.01618984274 [Google Scholar] [CrossRef] [PubMed]
[8]. Lim WK, Gurdeep GS, Norain K, Melioidosis of the head and neckMed J Malaysia 2001 56(4):471-76. [Google Scholar]
[9]. Srikanth P, Rathna M, Karthik RN, Jayaprakash B, An unusual site for melioidosis: parotid glandInternational journal of Scientific Research 2015 4(11)ISSN No2277-8179 [Google Scholar]
[10]. Raja NS, Ahmed MZ, Singh NN, Melioidosis: an emerging infectious diseaseJ Postgrad Med 2005 51:141-44. [Google Scholar]
[11]. Currie BJ, Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatmentSeminar on Respir Crit Care Med 2015 36:111-25.10.1055/s-0034-139838925643275 [Google Scholar] [CrossRef] [PubMed]
[12]. Rebecca L, Susan G, Rosemarie A, Prasith B, David DB, Allen CC, Workshop on treatment of and postexposure prophylaxis for Burkholderia pseudomallei and B. mallei, Infection. 2010Emerging Infectious Disease Journal 2012 18(12) [Google Scholar]