Intensive Care Units are the principle component of modern medicine where critically ill patients are taken care of [1]. However, most of the patients develop nosocomial infections as a part of ICU admission. Nosocomial pneumonia is defined as manifestation of pulmonary infection after 48 hours of hospital admission which can also be attributed to any procedures done to the patient [2]. It depends on the microbiological spectrum of individual ICU or can be due to any invasive or diagnostic procedures the patient undergoes in the ICU [1].
Among the ICU patients Hospital Acquired Pneumonia (HAP) and Ventilator Associated Pneumonia (VAP) remain the most cardinal causes of morbidity and mortality regardless of the recent advances in antimicrobial therapy, preventive measures and supportive care [3-7]. HAP is defined as pneumonia that occurs 48 hours or more after admission, which was not incubating at the time of admission [3,5]. As per the severity of hospital acquired pneumonia it can be decided whether the patient can be treated in wards or ICU. Pneumonia that occurs after 48-72 hours of intubation is referred to as ventilator associated pneumonia [4,5].
According to a study done by Richards MJ et al., on nosocomial infections in medical ICU, 25% of the ICU infections were HAP which accounted to more than 50% of the antibiotics demand in the hospital [8]. The standard of ICU and the different diagnostic methods used and precautions taken for the same are some of the factors that affect the development of VAP, which is almost 9-27% of all the intubated patients [1,9-11].
Intensive care units use present day contemporary medicines for the care and monitoring of patients, ventilatory supports and the most potent antibiotics to fight infections [1]. Multidrug resistant bacteria are a major threat in clinical practices especially in hospitals and their ICUs where patient care should be of prime concern. The drug resistant nature of pathogen and its unusual and unpredictable susceptibility patterns makes empirical and therapeutic decisions even more difficult. Treatment of nosocomial infections always remains a dilemma as the clinical presentation and the microorganisms causing the pneumonia will vary with the immunity of the patients and different institutions. Hence, there is need for early diagnosis and management of these patients to decrease morbidity and mortality. The decision of treatment on the basis of clinical culture result remains in the hands of the clinician. The current management of nosocomial pneumonia is based on the studies from western countries which may not be helpful for Southern Indian population. The present custom based ICU study for the Southern Indian population will be helpful in treating patients better and helps to customise a protocol for treatment of nosocomial pneumonia. Here the aim of the study was to find out the most common microorganism with pulmonary involvement in the ICU setup and their sensitivity patterns to the antibiotics by doing BAL.
Materials and Methods
The present study was a retrospective study conducted with the data collected between a one year period from January 2017 to December 2017 conducted at a tertiary care centre in Chennai, India, Saveetha Medical College and Hospital. Ethical Committee and Institutional Clearance was obtained (Registration number: 004/02/2017/IEC/SU). The study included 92 BAL samples taken from all consecutive patients referred with suspicion of pneumonia in the ICU of present hospital. Since, ICU cases are not regular cases, sample size was calculated by consecutive cases which was admitted and referred.
Inclusion criteria was new or progressive infiltrates on chest roentgenogram 48 hours or more after ICU admission with or without ventilatory support along with fever, purulent secretions, raising total counts and ESR levels. Patients on drug therapy, pre existing lung diseases and other comorbid conditions like diabetes were included.
Exclusion criteria was active Pulmonary Tuberculosis, Chronic Kidney Disease, Pulmonary oedema, Recent cardiac manifestations.
Bronchial wash was done with the help of fibreoptic bronchoscope under local anaesthesia (transtracheal). Around 10-30 mL of sterile normal saline was instilled into the infected lung lobe/bronchopulmonary segments. Instilled saline was suctioned back and collected into sterile containers. Collected samples of 92 patients were sent to microbiology laboratory of the hospital for identifying the microorganism using agars like blood agar, chocolate agar and MacConkey’s agar and their sensitivity to the antibiotic spectrum by an automated machine (Vitek).
Outcome measures of the study were identifying the sensitivity pattern of microorganisms so that further hospital based ICU admission will be treated with the new protocol. This will help in improving the quality of treatment care and reduce the morbidity of the patients.
Statistical Analysis
All basic statistical calculations like percentage calculation, development of graphs was done using spread sheets.
Results
The study included patients with the age range of 22-65 years with a mean age of 44.5 years. Males were 63 and females 29. Out of the 92 patients, 56% were diabetic, 35% had pre-existing lung diseases and 9% were already on drug therapy [Table/Fig-1].
| Demographic data |
|---|
| Age range | 22-65 years |
| Mean age | 44.5 years |
| Gender distribution |
| Males | 63 (68%) |
| Females | 29 (32%) |
| Concomitant infections |
| Diabetes mellitus | 56% |
| Pre-existing lung diseases | 35% |
| On drug therapy | 9% |
Out of the 92 BAL specimens, 58 samples were taken from intubated patients and 34 from non-intubated patients. Microbiological growth was seen in 84 samples while eight samples showed no growth. Out of the 84 positive samples 54 were from patients who were on mechanical ventilation and 30 from patients who were not on ventilators.
The most common bacteria found out from the 84 BAL specimens were Acinetobacter baumanii which accounted to 36 samples (39%) of the entire sample size. The second in line was Klebsiella pneumonia infecting 28 samples accounting to 30%. Pseudomonas aeruginosa was the third commonest with 12 samples accounting to 13%. Only 8 (9%) samples yielded no growth out of which 2% were patients on mechanical ventilation [Table/Fig-2].
Percentage yield of each bacteria in 92 BAL specimen (n=92).
| Organism | n (%) |
|---|
| Acinetobacter baumanni | 36 (39%) |
| Klebsiella pneumoniae | 28 (31%) |
| Pseudomonas | 12 (13%) |
| Escherichia coli | 3 (3%) |
| Streptococcus pneumonia | 2 (2%) |
| Coagulase neg Staph aureus | 2 (2%) |
| Enterobacter species | 1 (1%) |
| No growth | 8 (9%) |
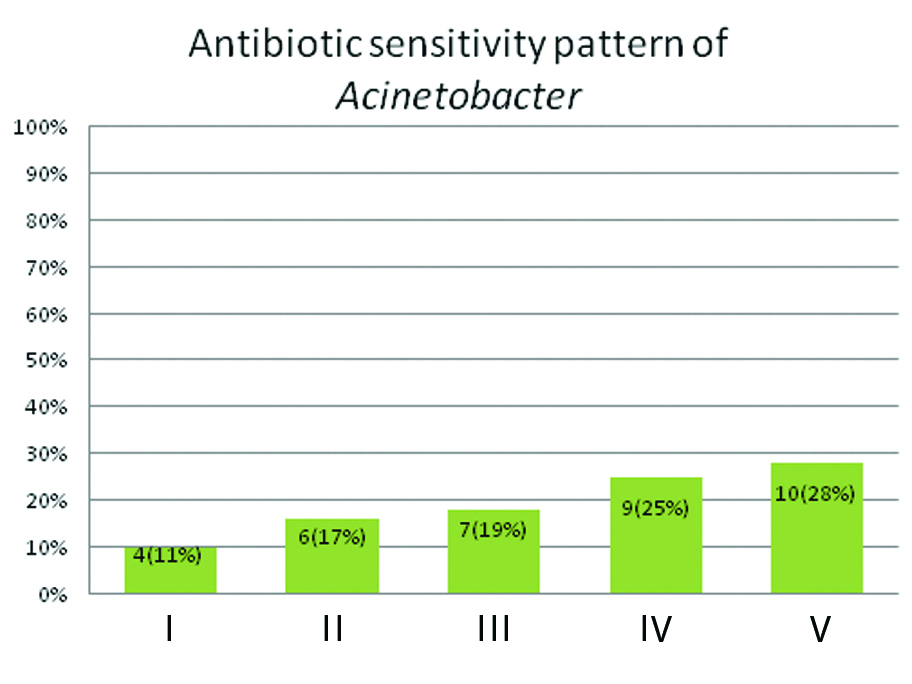
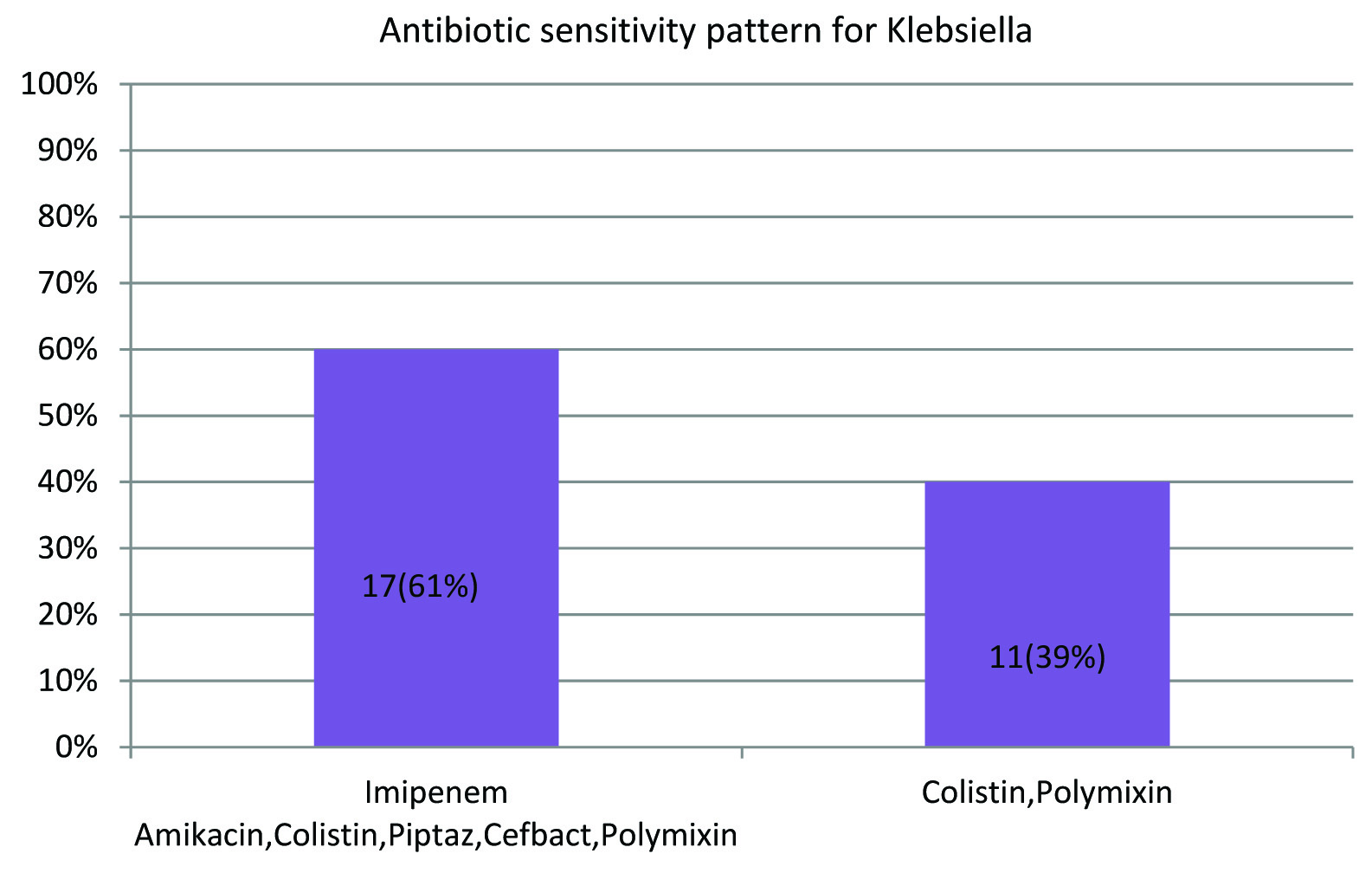
Among the 58 samples taken from intubated patients, 62% which is 36 samples was found to be Acinetobacter. Klebsiella accounted to 26% that is 15 samples, Pseudomonas was 9% (5 samples) and 3% (2 samples) showed no growth in the culture media. The remaining 34 samples taken from patients who were not on mechanical ventilation showed a predominance of Klebsiella organism with 13 samples (38%), Pseudomonas accounted to 7 samples (21%) while Streprococcus pneumoniae and Staphylococcus aureus was found to 6% which is two samples each. Escherichia coli was 3 samples (9%), Enterobacter species was seen in a single sample (3%). Around 6 samples (17%) of the patients showed no growth in the BAL culture [Table/Fig-3,4]. Comparing the antibiotic spectrum, the entire 36 samples of Acinetobacter species showed sensitivity for Colistin while 75% (27 samples) for Polymixin, 64% (23 samples) for Tigecycline and 47% (17 samples) for Imipenem. About 28% (10 samples) of the Acinetobacter were sensitive to all the commonly used antibiotics. Around 75% (27 samples) were sensitive to both Colistin and Polymixin, 24% to Colistin, Tigecycline and just 10% to all the three antibiotics. Around 60% (17 samples) of the Klebsiella pneumoniae was sensitive to the common broad spectrum antibiotics while 40% (11 samples) were sensitive only to Colistin and Polymixin. Rest of the microbiological organisms including Pseudomonas aeruginosa were sensitive to all the antibiotics [Table/Fig-5,6]
Distribution of microorganisms in patients on mechanical ventilation (n=58).
| Organism | n (%) |
|---|
| Acinetobacter baumanni | 36 (62%) |
| Klebsiella pneumonia | 15 (26%) |
| Pseudomonas | 5 (9%) |
| No growth | 2 (3%) |
Distribution of microorganisms in patients not on mechanical ventilation (n=34).
| Organism | n (%) |
|---|
| Klebsiella pneumonia | 13 (38%) |
| Pseudomonas | 7 (21%) |
| Escherichia coli | 3 (9%) |
| Streptococcus pneumonia | 2 (6%) |
| Coagulase neg staph aureus | 2 (6%) |
| Enterobacter species | 1 (3%) |
| No growth | 6 (17%) |
Antibiotic sensitivity pattern of Acinetobacter.
I: Colistin, Polymixin, Tigecycline; II: Colistin, Polymixin; III: Colistin, Imipenem, Polymixin; IV: Colistin, Tigecycline; V: Colistin, Polymixin, Tigecyclin, Imipenem, Amikacin, Piptaz, Cefbact
Antibiotic sensitivity for Klebsiella pneumonia.
Discussion
The study aims at finding out the most common microorganism with pulmonary involvement in the Intensive care unit setup with the changing trends of sensitivity patterns of microorganisms to the antibiotics by doing BAL.
In the present study, BAL reports of 92 patients were collected for assessing the aetiological agent for the pneumonia and their antibiotic sensitivity. According to the studies conducted by Chastre J et al., and Woske HJ et al., identification of microorganism was proven to be best achieved by BAL samples and protected brush specimens in patients diagnosed with pneumonia [11,12]. A study done by Johanson WG et al., on the bacteriologic diagnosis of nosocomial pneumonia following prolonged mechanical ventilation, he compared different specimens like tracheal aspirates, BAL, Protected-Specimen Brush (PSB) samples, and direct lung aspirates with cultures of lung homogenates with histological findings of intubated baboons and showed that BAL contributed the precise outlook of the bacterial burden of pulmonary infections [13]. Moreover, the American Thoracic Society and the infectious diseases society of America have issued evidence based guidelines that support the use of quantitative culture of bronchoscopically collected lower respiratory tract secretions for the aetiological diagnosis of VAP [14]. Such interventions facilitate early medical interventions by initiating appropriate antibiotic regimens or by indicating the need to search for an alternative cause when findings are negative.
Another study by Chastre J et al., compiled microbiological data of 24 published studies that used bronchoscopic diagnostic method to confirm 1689 cases of VAP in which they found that overall Gram negative bacteria represented 58% of isolates and Gram positive cocci made up to 35% [9]. A similar study by Fagon JY et al., about the prevalence of specific pathogens responsible for hospital acquired pneumonia including VAP also showed overall Gram negative bacteria as the major part of isolates [15]. These findings were similar to present study in which predominant microorganisms were gram negative (Acinetobacter, Klebsiella, Pseudomonas, E.coli and Enterobacter) accounting to about 80 out of 92 patients which is 87% of the entire sample size while gram positive organisms (Streptococcus pneumonia and Staphylococcus aureus) were only around 4%.
Acinetobacter has surpassed all the other bacteria in present study with 39% followed by Klebsiella pneumonia with 31% and Pseudomonas aeruginosa with 13%. Acinetobacter were found only in patients with mechanical ventilation in present study. It was found that most of the patients in mechanical ventilation were on corticosteroid therapy, immunocompromised state, pre-existing lung diseases and prior antibiotic therapy. Around 9% had no growth which can be attributed to administration of antibiotics prior to the procedure. Klebsiella pneumonia and Pseudomonas spp. was found in 26% and 9% of the intubated patients respectively.
According to the study conducted by Ahmed W et al., on microorganisms related with VAP and their antibiotic sensitivity pattern Acinetobacter baumanii outstripped the other pathogens as the leading cause of VAP [16]. As per the study by Gupta V et al., on prevalence of multidrug resistant pathogens and their antibiotic susceptibility pattern maximum isolates were Acinetobacter spp. followed by P. aeruginosa [17]. Rashid N et al., also reported Acinetobacter baumanii the most common pathogen among children followed by Pseudomonas aeruginosa [18]. Similar findings were noted in present study also. A study in Saudi Arabia also showed similar results with the highest number of cases being reported for Klebsiella pneumoniae, followed by Pseudomonas and then Acinetobacter [19]. A study of 753 cases done in a community hospital by Bapcock HM et al., compared causative agents for VAP and found that the common isolates were Pseudomonas, Acinetobacter and Staph.Aureus [20]. According to Chastre J et al., the most common pathogen was Gram negative E.coli, Pseudomonas aeroginosa and Staph. Aureus [9].
Antibiotic resistance is increasingly prevalent among the organisms isolated from ICU patients. In present study, among Acinetobacter high-level resistance was seen to Ciprofloxacin-100%, Amikacin, Piperacillin-Tazobactum, Cefbact was 72%. Colistin was found to be susceptible among all the organisms (100%) isolated while Polymixin to 75% of the cultures. Imipenem and Tigecyline showed 47% and 53% sensitivity each. In present study, the next predominant isolate was Klebsiella pneumonia which showed 60% sensitivity to the common broad spectrum antibiotics while 40% was sensitive to only Colistin and Polymixin. Among Pseudomonas species and others like E.coli, Enterobacter, Streptococcus pneumonia and Staphylococcus aureus the isolates were most susceptible to all the broad spectrum antibiotics.
In Spain, a study conducted by Picazo JJ et al., on antimicrobial resistance surveillance found Acinetobacter spp., with resistance rates of 45% for carbapenem, 70% for Piperacillin-Tazobactum and 35% for Amikacin [21]. Comparing different studies, the incidence of multidrug pathogens, Acinetobacter was found to be 37.5% by Golia S et al., [22], 84.5% in the study done at Karnataka and 75% in a study at Lucknow among VAP isolates [23,24]. In a study conducted in Europe, the non-susceptibility rates for P. aeruginosa have been reported to increase to about 20% for Carbapenems, 25% for aminoglycosides and about 8% for Piperacillin-Tazobactum [25].
On detailed analysis of these studies it is observed the rising emergence of resistant strains of Acinetobacter, Klebsiella and Pseudomonas globally. In such cases of highly resistant strains to most of the frequently used broad spectrum antibiotics, Colistin/Polymyxin B remains the last option for treatment. Doctors and paramedics have to be trained in proper hygiene techniques and aseptic precautions for all therapeutic and diagnostic procedures done, can prevent nosocomial infections to an extent. Simple techniques such as hand washing, placing the patient in semi recumbent position and avoiding excess sedation can prevent nosocomial pneumonia to a certain level. Noninvasive ventilation was found to be associated with reduced rates of infection and should be considered in patients who are conscious, haemodynamically stable and not moribund.
Limitation
The study had some limitations as the present study was a referral based study which included filtered population, these results may not be applicable to general population. Larger sample size yields better and more accurate results compared to smaller sample size. Latest antibiotics which are under trial/formulation have to be tested again to determine their sensitivity among ICU pathogens and to find out the efficacy of the antibiotics.
Conclusion
In an ICU setup, nosocomial infections are a major threat especially VAP showing increasing levels of multidrug resistance pathogens. As per the study Acinetobacter was the most common organism isolated from ventilator associated pneumonia patients with a high percentage of resistant strains followed by Klebsiella and Pseudomonas. Although all the microorganisms were sensitive to high level antibiotics only a few were sensitive to the common broad spectrum antibiotics.
Hence, with the knowledge of the commonest organism isolated along with their resistance pattern to the antibiotics, each institution can phrase their own antimicrobial policy for treatment of nosocomial pneumonia especially Ventilator associated pneumonia depending on their local evidence.