The effect of occupational hazards on the reproductive system has been the primary focus of various research activities throughout the world. It has been noted that certain toxins produce hormonal alterations resulting in structural changes in reproductive organs [1]. The National Institute of Occupational Safety and Health (NIOSH) has identified certain harmful chemicals induced infertility (following occupational exposure), a potential area of research study [2].
Formaldehyde is an organic carbon compound commonly employed for embalming cadavers, preservation of biological specimens and as a disinfectant. Apart from being an occupational hazard, it is also a common source found indoors from unvented fuel burning appliances, kerosene heaters, baby shampoos, cosmetics and from pressed wood products [3,4]. The general population is exposed to minimal concentrations (0.1 mg/m3) of formaldehyde [5] in their everyday life.
It has been suspected of being a toxicant affecting the reproductive system, especially males [6]. The extent of damage varies especially depending on the rate of metabolism. Formaldehyde induces cytotoxicity in air passages, skin, liver and various other organs. The exposure of this compound through inhalation affects a wide range of population including medical professionals, laboratory technicians, and funeral home employees, students exposed to cadavers, cosmetologists and industrial workers [7]. Hence it is mandatory to evaluate the toxic effects of this compound on reproductive organs.
There are only a few studies done, to show the toxic effects of formaldehyde, in our country.
Chowdhury AR et al., discussed the effects of formaldehyde on rat testis following intra peritoneal injection of the compound [8]. The risks of a negative outcome on the organs may be modified by genetic variation in detoxification activities [9]. Hence the potential toxic effects of formaldehyde on testis were evaluated in rats under our tropical conditions. This is done by analysing the microscopic changes following formaldehyde exposure by inhalation in the present study.
Materials and Methods
This animal experimental study was conducted at the Department of Anatomy, Government Kilpauk Medical College, Chennai after obtaining approval from Institutional Animal Ethics Committee (Reg. No–375/GO/ReBi/S/01/CPCSEA). The duration of the study was for three months from August 2016 till the end of October 2016. The animals required were provided by the Animal House at our institution. Twenty adult male rats-Rattusnorvegicus, weighing 180+20 gm were used. They were maintained at standard laboratory conditions such as room temperature 25-30°C, light-dark cycle for 12 hours each with access to food and water ad libitum.
Animal grouping: The rats were divided into five groups of four each. The control group was not exposed to formaldehyde. The aqueous solution of formalin (37% of formaldehyde in water) that is commonly employed in embalming procedure and in the preservation of various viscera in the Department of Anatomy was used in this study. It contains 37 gm of formaldehyde in 100 mL of water (weight/volume).
Study design: The experiment groups were divided into E1a, E1b, E2a and E2b according to the level and duration of exposure to formaldehyde [Table/Fig-1]. One ppm equals 1 mg/kg. The conversion of weight by volume to Parts Per Million (ppm) was validated using the given formula [10].
Level and duration of formaldehyde exposure.
| S No. | Experiment group | Level of formaldehyde exposure | Total duration of exposure | Time of exposure in a day |
|---|
| 1. | E1a | 1.5 ppm | 7 weeks | 6 hours |
| 2. | E1b | 1.5 ppm | 12 weeks | 4 hours |
| 3. | E2a | 3.0 ppm | 7 weeks | 6 hours |
| 4. | E2b | 3.0 ppm | 12 weeks | 4 hours |
In the exposure groups, the alphabet ‘E’ denotes experiment group, ‘1’ represents 1.5 ppm of exposure, ‘2’ denotes 3.0 ppm, ‘a’ represents 6 hours of exposure and ‘b’ denotes 4 hours of exposure.
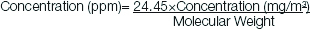
The molecular weight of formaldehyde is 30.03 grams per mole. In the present study, 5 mL of formalin solution was diluted in one liter of water.

5 mL dissolved in 1 L of water=1.85 mg/L or 1.85 mg/m3.
Using the conversion formula=24.45×1.85/30.03 equals 1.5 ppm.
Hence, 5 mL of formalin solution dissolved in 1 L of water is used for 1.5 ppm exposure groups (E1a, E1b) and 10 mL of the same was used for 3 ppm exposure groups (E2a, E2b). Alternately, Kaden DA et al., [11] had directly given the conversion factors as 1 mg/m3 equals 0.814 ppm [11]. Hence, 1.85 mg/m3 equals 1.505 ppm.
Formalin treatment procedure: The experiment group animals were placed in standard cages of 38×20×18 cm size (four animals per cage). The solution was placed in a plastic container with holes in it and kept at the corner of cages. The exposure was carried out in a closed chamber of 55×36×34 cm size. The chamber was modified with openings for light and ventilation at different levels. The longer duration of exposure in a day was because the half-life for the metabolism of formaldehyde to formic acid is about one hour [11] and it is absorbed rapidly in rodents [12,13]. Hence, observed the changes as a result of increased exposure time in a day.
Follow-up procedure: At the end of intended exposure period, the rats were euthanised by administering over dose of chloroform. On cessation of respiration, transcardiac perfusion of 10% formal saline was performed. The testes were dissected from the surrounding structures, observed for gross morphological changes, weighed, measured and fixed in freshly prepared 10% formalin.
Tissue processing: Following fixation in 10% formalin for 24 hours, tissue processing steps like dehydration, clearing, paraffin embedding and serial cut sections in a rotary microtome (6 μm) was done. Then they were stained using haematoxylin and eosin stains for microscopic evaluation.
Statistical Analysis
Data were entered in Microsoft excel spreadsheets. One-way ANOVA with Tukey’s post-hoc test and Unpaired t-test was used for comparison between groups. This was done using GraphPad Instat version 3.1 for windows (GraphPad Software, San Diego, California, USA). The p-value less than 0.05 were considered statistically significant. The values measured were expressed as mean±standard deviation.
Results
The testis was observed for any morphological changes between the control and the experiment group. The total body weight in the control group was 195±6.37 gm. There was a significant reduction in the total body weight of experiment group rats which measured 157±3.71 gm (p<0.0001-Unpaired t-test). The morphometric data related to weight, length and width of rat testis is shown in [Table/Fig-2].
Morphometric data related to testis following formaldehyde inhalation.
| S No. | Group | Testis weight (gm) | Testis length (mm) | Testis maximum width (mm) |
|---|
| 1. | Control | 0.767±0.22 | 17.97±0.87 | 11.34±1.23 |
| 2. | E1a | 0.731±0.31 | 16.37±1.16 | 11.05±0.56 |
| 3. | E1b | 0.701±0.45 | 16.22±0.65 | 11.04±0.28 |
| 4. | E2a | 0.667±0.66 | 14.89±1.84 | 10.58±1.11 |
| 5. | E2b | 0.605±3.21 | 13.99±2.24 | 10.10±3.43 |
There was a minimal decrease in the weight and width of the testis in the experiment group in comparison to the control group (p>0.05) however there was a significant progressive reduction in the length of testis (p-value<0.0001) with increasing concentration of formaldehyde exposure.
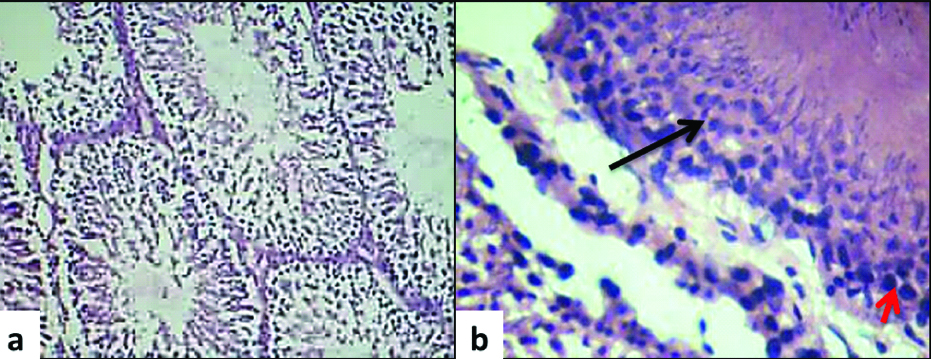
The histologic appearance of the control group is shown in [Table/Fig-3a,b]. The control group showed normal architecture of seminiferous tubules with sertoli cells, spermatogenic lineage of cells, with minimal connective tissue around tubules. The histological analysis of the experiment group is summarised in [Table/Fig-4]. There was an increased accumulation of lymphocytes in the connective tissue in all the experiment groups. The increased time of exposure in a day (E1a and E2a) was not found to have an adverse impact when compared with the total increase in duration of exposure groups (E1b and E2b). The E1b group showed increased necrosis with minimal peritubular fibrosis in comparison with E1a group. The E2b group presented with no necrosis, but with much increased peritubular fibrosis. Likewise, there was absent spermatogenesis in E2b group whereas E2a group presented with hypospermatogenesis. Apart from these changes, there was sertoli cell crowding and Leydig cell hyperplasia in E2b group. The interstitial cells were normal in all the groups except E2b group which presented with interstitial cell hyperplasia. The microscopic changes are tabulated in [Table/Fig-4].
Light microscopic image from a control group rat testis a) normal architecture of seminiferous tubules (10X, H&E); b) black arrow pointing normal spermatogenic lineage of cells (spermatogonia lying around the periphery phasing out to primary spermatocytes, secondary spermatocyte, spermatids and mature sperm as we proceed towards the lumen of the tubule) and red arrow showing sertoli cell within a tubule (40X, H&E).
Histologic changes following haematoxylin and eosin staining following formaldehyde exposure.
| S No. | Experiment Group | Focal Tubal Necrosis | Peritubular Connective Tissue | Spermatogenesis | Sertoli cells | Interstitial cells of Leydig |
|---|
| 1. | E1a | Minimal focal tubular necrosis | No peritubular fibrosis | Focal tubular atrophy | Sertoli cells normal | Interstitial cells normal |
| 2. | E1b | Increased focal tubular necrosis | Minimal peritubular fibrosis | Focal tubular atrophy with hypospermatogenesis | Sertoli cells normal | Interstitial cells normal |
| 3. | E2a | Minimal focal tubular necrosis | Minimal peritubular fibrosis | Focal tubular atrophy with hypospermatogenesis | Sertoli cells normal | Interstitial cells normal |
| 4. | E2b | No necrosis | Much increased peritubular fibrosis | Absent spermatogenesis | Sertoli cells crowding | Focal hyperplasia |
The images of histological changes in the exposure groups are shown in [Table/Fig-5,6,7,8 and 9]. The significant changes noticed in the microscopic analysis of histological preparation encompasses an inverse relationship between tubular necrosis and peritubular fibrosis, gradual arrest in maturation of sperms from hypospermatogenesis to absent spermatogenesis, sertoli cell crowding, progression of peritubular fibrosis and focal hyperplasia of interstitial cells of Leydig with increasing concentrations and duration of formaldehyde exposure.
Light microscopic image (10X, H&E) belonging to E1a group showing focal tubular necrosis (arrows)
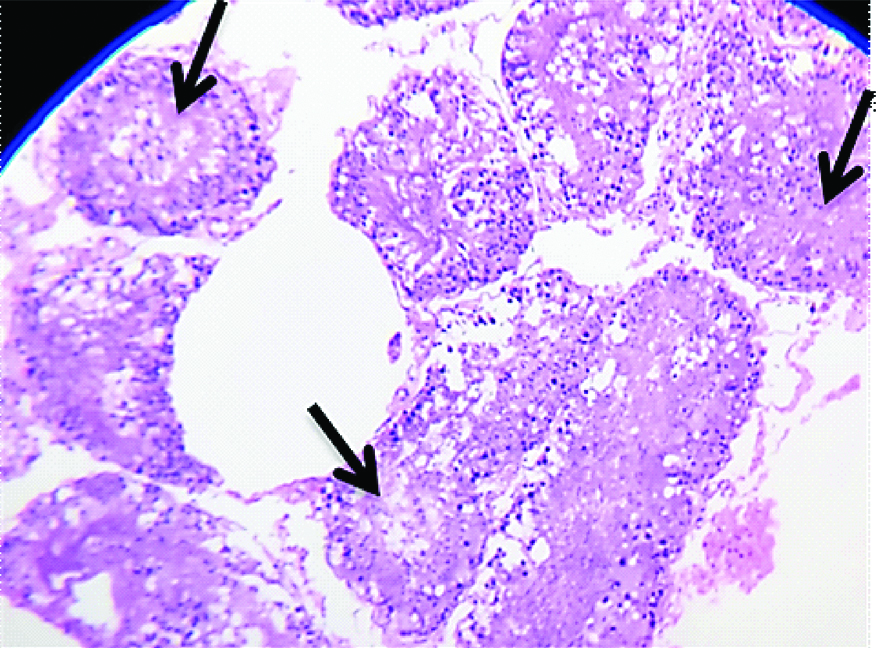
Light microscopic image (10X, H&E) belonging to E1b group showing minimal peritubular fibrosis (black arrow), focal tubular necrosis (red arrow) and architectural distortion of seminiferous tubules (yellow arrow).
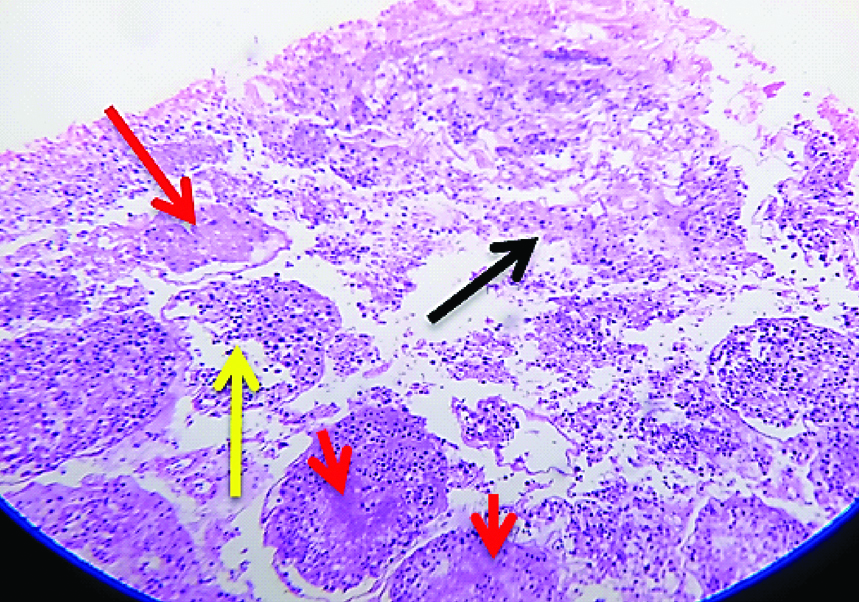
Light microscopic image (40X, H&E) belonging to E2a group showing a focal atrophied tubule with hypospermatogenesis.
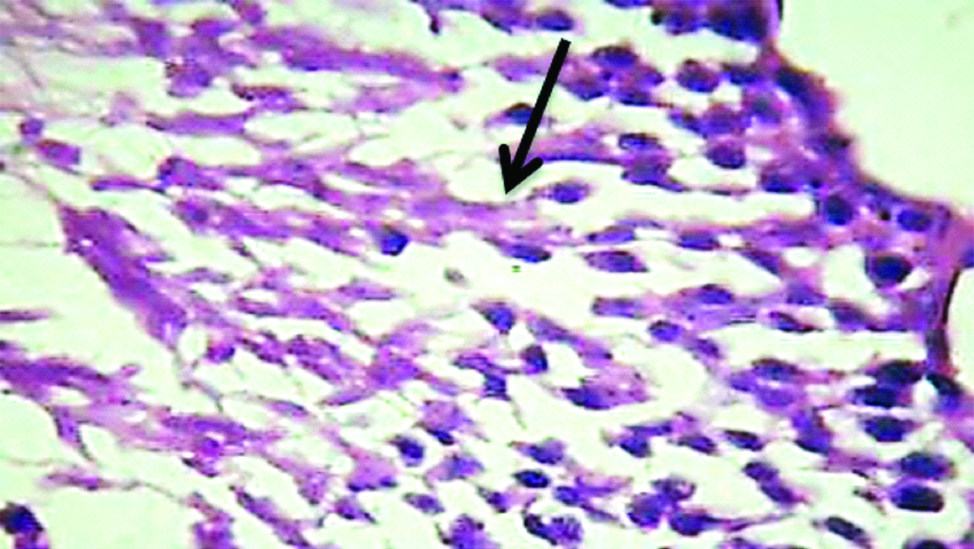
Light microscopic image (10X, H&E) belonging to E2b group showing: 1) Peritubular fibrosis; 2) Sertoli cell crowding; 3) Absent Spermatogenesis.
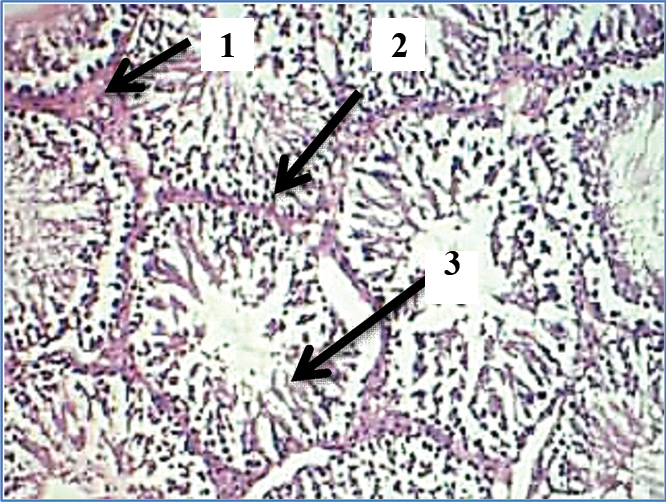
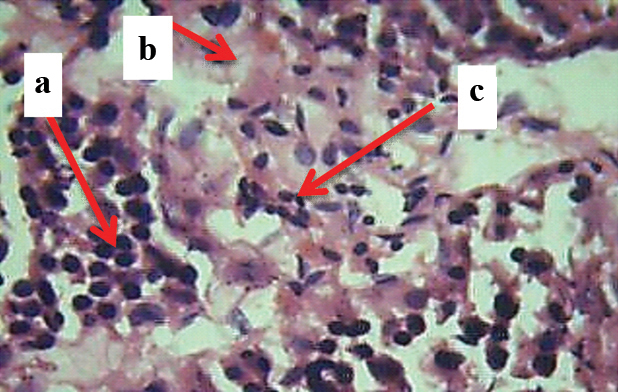
9-Light microscopic image (40X, H&E) belonging to E2b group showing: a) Sertoli cell crowding; b) peritubular fibrosis; c) Interstitial cell hyperplasia.
Discussion
Formaldehyde is a ubiquitous chemical compound which is recently classified as a potential carcinogen. It has also been suspected of causing increased fertility related problems [14]. The severity of damage induced depends on various factors such as level of exposure, individual sensitivity to the compound and genetic variation in metabolism. Hence, an attempt to study the effects of formaldehyde at varying concentrations on one of the male reproductive organ, testis was made in the current study.
The spermatogenesis cycle in Rattus norvegicus is around 50 days [15-18]. Hence, the changes in microscopic anatomy of testis following exposure were evaluated at the end of seven weeks (corresponding to the complete spermatogenesis cycle in Rattus norvegicus) for groups E1a and E1b. The observation such as hypospermatogenesis (decrease in germ cells) seen in E1b group is similar to a study by Golalipour MJ et al., [19]. In order to analyse the further progression of changes on prolonged exposure, the groups E2a and E2b were evaluated at the end of 12 weeks. There was definite worsening of histological changes including increased peritubular fibrosis and absent spermatogenesis in groups E2a and E2b.
The role of formaldehyde as a potential toxin exerting adverse outcome especially on the male reproductive system has been suspected for a long time [1]. The possible mechanism underlying the reproductive toxicity of formaldehyde is that the compound crosses the blood testis barrier and inflicts injury by increasing the reactive oxygen species thereby depleting the cellular glutathione levels and inducing oxidative stress [20]. Formaldehyde is an electrophile that can react directly with DNA, RNA and proteins and form crosslinks and adducts [21].
The changes in microscopic anatomy in the exposure group include focal tubular necrosis in the initial stages of exposure. With continued exposure hypoplastic germinal epithelium [22] with fewer mature spermatozoa, crowding of sertoli cells and arrest of spermatogenesis which are common features of testicular atrophy [23,24] were observed. Focal hyperplasia of interstitial cells of Leydig was also observed with prolonged exposure which appears to be due to cessation of spermatogenic activity and tubular atrophy [25]. This feature is in contrast to other studies which report decrease in the sertoli cells and Leydig cell population following formaldehyde exposure [26-28]. In conditions with severe depletion or absence of germ cells within the seminiferous tubule, the sertoli cells assume extensive junctional complexes along their contact surface with unusual interdigitations and appear entangled with each other [29]. Likewise, the Leydig cells were found as hyperplastic nodules in conditions of germ cell depletion in a seminiferous epithelium. This apparent increase was probably due to the atrophy of tubules [29]. This study possibly explains ‘sertoli cell crowding’ and Leydig cell hyperplasia with absent spermatogenesis in E2b group.
There was an increased fibrosis in the peritubular tissue which is also called the tunica (lamina) propria. The E2a and E2b groups expressed increased fibrosis compared to the low dose exposure group. There is evidence that this thickening due to fibrosis of peritubular tissue causes a reduction in the size of seminiferous tubules. This is a normal feature of aging. However, increased fibrosis in tunica propria in earlier stages is usually associated with infertility as a result of decrease in rate of sperm production [30].
The exact underlying molecular changes resulting in sertoli cell crowding and hyperplasia of Leydig cells need to be evaluated. This indicates that further evaluation of the biological mechanisms at the molecular level leading to morphological changes becomes mandatory. Since the study involved euthanasia of rats, only minimum number of rats (n=20) were provided by the Institutional Animal Ethics Committee.
Limitation
The formaldehyde exposure and the concentration in parts per million was calculated using the conversion formula. The exact concentration in parts per million of formaldehyde in air in real time following formalin solution exposure could not be calculated because of unavailability of the gas chamber with detector tube.
Conclusion
The study showed that exposure of even minimal doses of formaldehyde such as 3 ppm can have an adverse impact on the testis including focal tubular necrosis, peritubular fibrosis, finally leading on to arrest of spermatogenesis. Thus, further evaluation of the underlying mechanism resulting in these changes following formaldehyde exposure becomes necessary.
In the exposure groups, the alphabet ‘E’ denotes experiment group, ‘1’ represents 1.5 ppm of exposure, ‘2’ denotes 3.0 ppm, ‘a’ represents 6 hours of exposure and ‘b’ denotes 4 hours of exposure.