Ayurveda is an ancient traditional medicine system originated, evolved and practiced in India over thousands of years and its formulations have been used in therapeutics from the natural resources such as herbs, minerals, metals, etc., In which about 49 are herbo-minerals, and 110 are mineral formulations [1,2].
A specialised branch of Ayurveda, known as Rasashastra deals with the modified drug formulations in which the selected metals and minerals are used [3-7]. Bhasma is a well known potent inorganic preparation obtained from specific metals and minerals which are converted into compounds of carbonates or oxides etc. Its common usage in the ayurveda is proved to be successful in the treatment of certain illness and is considered to be popular because of its stability, lower dose and its ready availability [8]. Previous study on repeated administration of therapeutic dose level using Tamra Bhasma did not show any toxic effect or genetic aberrations [9]. However, Tamra Bhasma at five times of Therapeutic Dose (TED) produced changes in biochemical parameters such as there was an increase in the serum creatinine level. Total bilirubin level was found to be elevated. Kidney sections from Tamra Bhasma administered at five times of therapeutic dose has shown necrotic changes in the tubular epithelium, vacuolisation oedema in the interstial tissue and focal cell infiltration [9].
Jasada Bhasma is one of the zinc-based metallic preparations widely used in the alternative system of medicine in the treatment of certain illnesses related to zinc deficiency, throat, eyes, respiratory system, heart, digestive system and skin diseases such as acne, eczema, and psoriasis. Jasada Bhasma is useful in all types of fever and infections due to immune-modulatory effects. It improves the immune system and helps the body fighting off the disease [10-15].
The imperfect knowledge of understanding of traditional methods resulted in difficulty to reproduce authentic preparations. Jasada Bhasma is an ayurvedic preparation obtained by purification and calcination of zinc with herbal extracts. Various stages of its preparation are ‘Shodana’ (purification), ‘Jarana’ (trituration), and ‘Marana’ (calcination) [16]. All the preparations were proven therapeutically effective. But the presence of heavy metals always becomes a point of concern in the current scenario [17-19].
Considering above facts, research on evaluation of safety and efficacy of repeated administration of Jasada Bhasma formulations is required in the field of biomedical sciences. So, in the present study, biochemical, haematological and histopathological evaluation of toxicity which may result from the repeated administration of the Jasada Bhasma was carried out using the animal model.
Materials and Methods
In the present experimental study, Wistar albino rats were used with the body weight ranging from 150-225 gm. These animals were accustomed to the standard laboratory conditions of temperature (25±2°C), humidity (55-60%) and 12 hours day and night cycle during the study. The animals were provided with the rat diet and water ad libitum. The Institutional Animal Ethical Clearance was obtained prior the study with the reference number bearing SDMCA/IAEC/CPCEA/GI04/2011 from Srinivas Institute of Medical sciences, Mukka, Mangaluru, where the study was conducted for the period of three years nine months (September 2012-June 2016).
Test drug: Jasada Bhasma (a Zinc preparation) was used to assess the pathological and biochemical profile. The test drug was manufactured by a renowned expert in Rasashastra (a specialised branch of Ayurvedic Science).
Dose fixation: The human dose of Jasada Bhasma for therapeutic activity is 120 mg/kg/body weight [20]. The rat dosage was estimated by body surface area ratio by referring to the standard table of Paget and Burns [21]. From this, the rat dose was estimated to be 10.8 mg/kg (therapeutic dose) and 54 mg/kg body weight (five times of therapeutic dose). The test drug Jasada Bhasma was suspended in 0.5% Carboxymethylcellulose (CMC) in 20 mL of distilled water and based upon the body weight of animals (1 mL/100 gm body weight) was administered orally by using a catheter.
Study design: The experimental animals were categorised into three different groups on random selection basis. Each group comprised of 10 rats. Group-I animals were served as the control and received only 0.5% CMC as the vehicle. Group-II rats received Jasada Bhasma at a therapeutic dose (TED) 10.8 mg/kg, Group-III rats received five times of therapeutic dose (TED X5) 54 mg/kg. The test drug Jasada Bhasma was administered for 28 consecutive days. Following the last administration of the dosage, the blood sample was obtained for biochemical and haematological investigations. It is followed by the scarificing of all the animals and removal of some vital organs such as liver, kidney, spleen, brain, heart, lung, stomach, and jejunum for the histopathological examinations. The tissues from each specimen were stained with routine Haematoxylin and Eosin stain and examined under the microscope [22].
Efficacy and toxicity profile were evaluated by observing the effect of test drug on body weight, effect on biochemical and haematological parameters (vide infra).
The following haematological parameters were tested. The Haemoglobin concentration (Hb), Red Blood Cell (RBC) count, White Blood Cell (WBC) count/Total Leukocyte Count (TLC), Packed Cell Volume (PCV), Mean Corpuscular Volume (MCV), Mean Corpuscular Haemoglobin (MCH), Mean Corpuscular Haemoglobin Concentration (MCHC), Red Cell Distribution Width -Coefficient Of Variation (RDWCV), Red Cell Distribution Width- Standard Deviation (RDWSD) and platelet count.
Statistical Analysis
The result data were expressed in mean±SEM and the statistical analysis of variance (ANOVA) followed by Dunnett’s multiple comparison t-test was performed using GraphPad Instant version 3.5 software. The ‘p<0.05’ was considered as statically significant.
Results
The repeated administration of Jasada Bhasma for 28 consecutive days did not produce any significant change in the organs weight, and observed changes were comparable with that of normal control.
Among the measured haematological parameters, the MCHC was significantly (p<0.01) increased in comparison to the normal control group of Jasada Bhasma administered at the therapeutic dose and five times of therapeutic doses [Table/Fig-1].
Haematological parameters comparison between Jasada Bhasma therapeutic dose (JB-TED) and Jasada Bhasma five times of therapeutic dose (JB TEDX5) with that of the normal control group.
| Parameters | Control | JB TED | JB TED X5 |
|---|
| Hb (g/dL) | 15.2±0.44 | 15.65±0.50 | 15.51±0.48 |
| WBC (cells/microL) | 5516.67±826.81 | 8296.66±1210.9 | 7600±916.52 |
| RBC (millions/microL) | 7.55±0.27 | 7.54±0.15 | 7.64±0.21 |
| PCV (%) | 43.45±1.30 | 42.13±1.34 | 41.87±1.26 |
| MCV (fL) | 57.65±0.62 | 55.90±1.18 | 54.867±0.65 |
| MCH (pg/cell) | 20.12±0.28 | 20.68±0.44 | 0.28±0.30 |
| MCHC (g/dL) | 34.95±0.19 | 37.12±0.14** | 37.03±0.15** |
| RDWCV (%) | 12.55±0.20 | 12.78±0.28 | 13.38±0.20 |
| RDWSD (fL) | 25.87±0.59 | 25.72±0.77 | 26.12±0.36 |
| PLT (lakh/microL) | 7.01±0.13 | 6.59±0.24 | 6.53±0.43 |
{Data expressed in Mean±SEM, *p<0.05, **<0.01 in comparison to normal control- ANOVA
Hb: Haemoglobin; WBC: White blood cells; RBC: Red blood cells; PCV: Packed cell volume; MCV: Mean corpuscular volume, MCH: Mean corpuscular haemoglobin; MCHC: Mean corpuscular haemoglobin concentration; RDWCV: Red cell distribution width-coefficient of variation; RDWSD: Red cell distribution width-standard deviation; PLT: Platelet count}
Biochemical investigations revealed statistically significant (p<0.01) decrease in serum urea level but increase in serum creatinine levels in both TED and TED X5 Jasada Bhasma dose levels. However, the significant increase in the potassium level (p<0.01) at TED X5 level and increase in total protein (p<0.05) at TED level was observed when compared to normal control group [Table/Fig-2].
Results of Biochemical parameter with the comparison between Jasada Bhasma therapeutic dose (JB-TED) and Jasada Bhasma five times of therapeutic dose (JB TEDX5) with that of the normal control group.
| Parameters | Control | JB TED | JB TED X5 |
|---|
| Glucose (mg/dL) | 100.50±10.86 | 117.35±3.47 | 112.45±4.32 |
| SGOT (units/L) | 115.5±9.52 | 121.66±6.57 | 143.67±6.93 |
| SGPT (units/L) | 134±15.92 | 104.17±6.62 | 140.17±6.31 |
| ALP (IU) | 283.33±48.55 | 381.17±44.911 | 388.67±29.40 |
| Urea (mg/dL) | 57.16±3.25 | 31.1±93** | 28.70±0.80** |
| Creatinine (mg/dL) | 0.6±0.07 | 143±0.04** | 1.20±0.03** |
| Sodium (mEq/dL) | 139.33±0.50 | 139.17±0.60 | 138.17±0.40 |
| Potassium (mEq/dL) | 4.28±0.11 | 4.75±0.11 | 5.07±0.18** |
| Total protein (g/dL) | 5.33±059 | 6.47±0.12* | 6.27±0.06 |
| Direct Bilirubin (mg/dL) | 0.14±0.11 | 0.14±0.11 | 0.15±0.11 |
| Total Bilirubin (mg/dL) | 0.11±0.01 | 0.11±0.01 | 0.11±0.00 |
{Data expressed in Mean±SEM, *p<0.05, **<0.01 in comparison to normal control-ANOVA
SGOT: Serum glutamic oxaloacetic transaminase; SGPT: Serum glutamic pyruvic transaminase; ALP: Alkaline phosphatase}
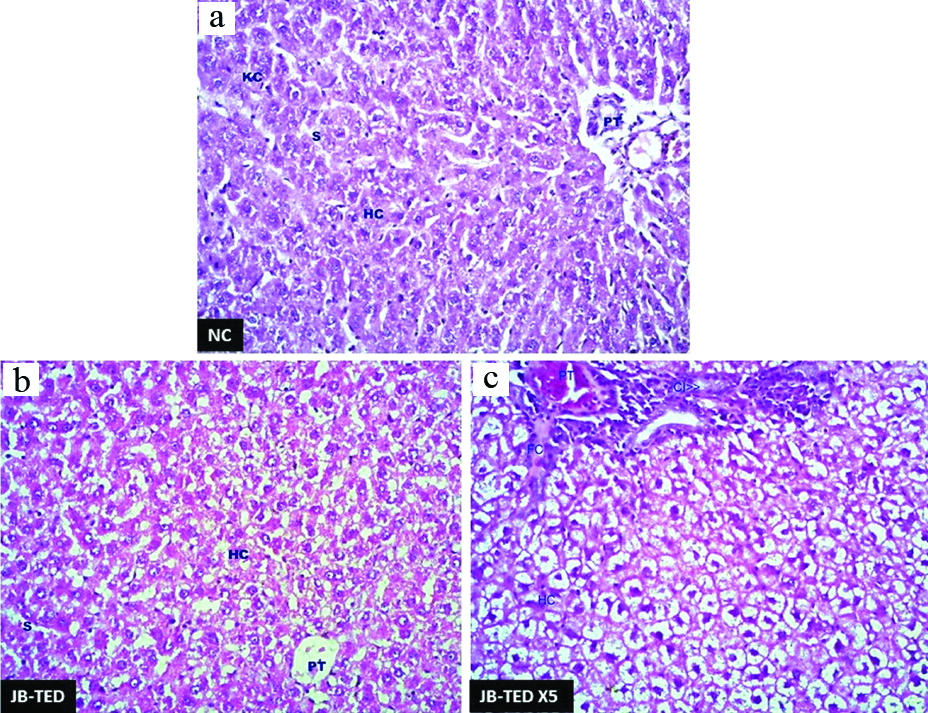
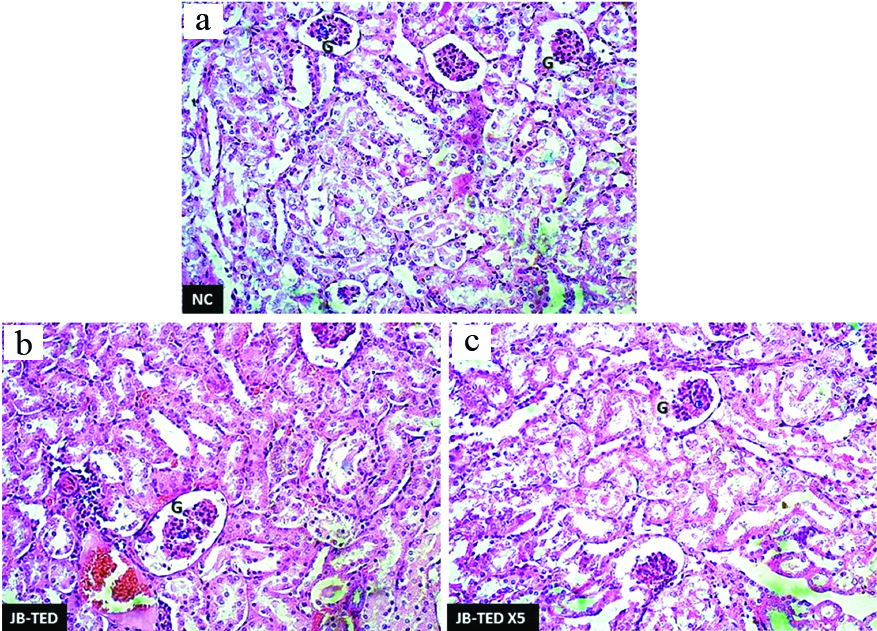
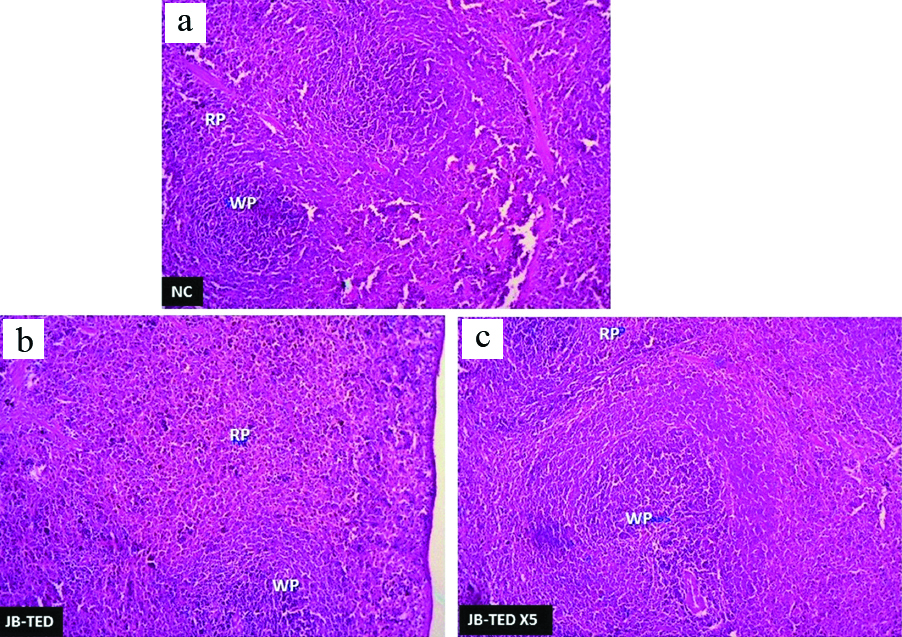
Histopathologically, the effect of the Jasada Bhasma on the tissues of brain, heart, lungs, jejunum, and stomach did not reveal any toxic changes and cytoarchitecture was almost normal as compared to control group. The liver tissues of Jasada Bhasma administered at both the dose levels showed mild to moderate sinusoidal dilatation and moderate diffused micro fatty changes [Table/Fig-3]. On the other hand, the sections of kidney showed necrotic changes in the tubular epithelium, vacuolisation oedema in the interstitial tissues and focal cell infiltration in both TED and TED X5 dose exposures of Jasada Bhasma [Table/Fig-4]. Jasada Bhasma therapeutic dose administered spleen sections showed more or less normal cytoarchitecture, whereas at five times of therapeutic dose a mild to moderate increase in the white pulp proportion was observed [Table/Fig-5].
H&E preparation showing the changes in liver tissues with the comparison of normal: (a) to that of exposure to Jasada Bhasma therapeutic dose; (b) and Jasada Bhasma five times of therapeutic dose (c) levels. {Hc: Hepatic cells; Kc: Kupfer cells; S: Sinusoids; PT: Portal triad; CI: Cell infiltration} 40X magnification.
H&E preparation showing the changes in kidney tissues with the comparison of normal: (a) to that of exposure to Jasada Bhasma therapeutic dose; (b) and Jasada Bhasma five times of therapeutic dose; (c) levels. {PCT- proximal convoluted tubules, DCT- distal convoluted tubules} 10X magnification.
H&E preparation showing the changes in spleen tissues with the comparison of normal: (a) to that of exposure to Jasada Bhasma therapeutic dose; (b) and Jasada Bhasma five times of therapeutic dose; (c) levels {RP: Red pulp; WP: White pulp} 4X magnification.
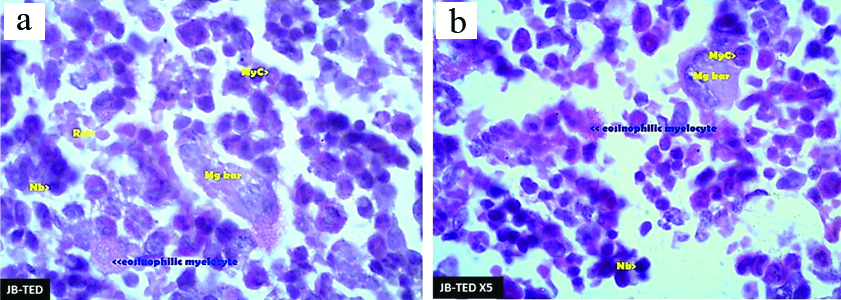
The bone marrow sections of Jasada Bhasma administered at therapeutic dose group has shown moderate cellularity in comparison to normal control. No cells with micronuclei found. Megakaryocytes and cells of eosinophilic lineage were present in higher number in contrast to the normal control group. Few reticulocytes were seen. Jasada Bhasma administered at five times of therapeutic dose, group cellularity was comparatively decreased in comparison to normal control group. While the myelomonocytic cell lineage was abundant in comparison to erythroid cell lineage, megakaryocytes were within normal range. Eosinophilic lineage was less abundant. Similar to TED, few reticulocytes were also observed in TED X5 levels of exposed bone marrow sections, and no cells with micronuclei were found [Table/Fig-6].
Romanowsky stain preparation of bone marrowshowing thechanges in the toxicity following the exposure to JasadaBhasma therapeutic dose: (a) and Jasada Bhasma five times of therapeutic dose; (b) levels. {Myc- Myelocytes, Mgkar- Megakaryocytes, Nb- Normoblasts, Ret- Reticulocytes} 100X magnification.
Discussion
Therapeutic use of Jasada Bhasma with it’s most important anti-diabetic activity in both Type-1 and Type 2 diabetes mellitus has been validated before [23]. Zinc is responsible for synthesis, storage, secretion of insulin in pancreas [24]. It prevents the degradation of insulin hexamers and also improves the binding of insulin to its receptors [25]. In the present research study, the overall toxicity profile of zinc formulated Jasada Bhasma administered in two levels of the therapeutic dose was assessed haematologically, biochemically and histopathologically.
Among the evaluated haematological parameters, the Jasada Bhasma administered at both dose levels exhibited a mild increase in total leucocyte count, but it was statistically insignificant. However, the MCHC was increased remarkably with the statistically significant increment in comparison to normal control group. The MCHC together with other associated red cell indices are the indicators reflecting the size and haemoglobin content of red cells that have been used to aid in the differential diagnosis of anaemia [26]. The elevation in the MCHC (normal range is 28-36 gm/dL) could be due to spherocytosis or autoimmune haemolytic anaemia (it is a type of RBC that contains an abnormal amount of haemoglobin). If the haemoglobin is not stable, this can cause remarkable raise in the MCHC. This elevation may also rise in case of the folic acid or vitamin-B12 deficiency and in other rare haematological disorders [27]. Repeated administration of test drug Jasada Bhasma could have an adverse effect on haemoglobin stability which may be responsible for the elevated MCHC level.
Mild increase in total leucocyte count usually indicates the inflammation and infectious state or disease of bone marrow. In the present study, Jasada Bhasma administered at both the dose levels, shown a mild increase in WBC; it might be due to inflammatory changes seen in some tissues. But comparatively, the reduced WBC in those groups administered with five times therapeutic dose can be suggested it as a limited nature change not important from the pathological point of view. Thus, the overall effect of test drug Jasada Bhasma on the haematological parameters does not have significant potential to cause blood-related toxic effects [28].
Jasada Bhasma treated groups showed dose-dependent significant changes in biochemical parameters recorded. While the changes are observed in serum urea, creatinine and total protein at the therapeutic dose level, serum potassium level showed the significant increase at five times of therapeutic dose level.
Urea and creatinine are end products of metabolism of nitrogenous materials in the body. In the present study, the serum urea level was diminished, and the creatinine level was elevated upon the exposure to test drug. The lowered serum urea level may be due to its decreased absorption. The elevation in serum creatinine level may be due to its impaired renal clearance. It may be elevated due to higher consumption of dietary proteins. Nevertheless, it is pertinent to mention here that after drug administration raised serum protein level was observed for the unknown reason. Because of the contradictory nature of the results obtained based on serum urea and creatinine level measurement, it is difficult to infer the conclusions precisely. However, it is prudent to monitor renal function in persons receiving these drugs on a long-term basis.
Jasada Bhasma administeration at therapeutic dose showed a significant elevation of serum total protein level. It indicates that the test drug might have a role in inducing inflammatory changes in repeated dose. However, the exact reason is not known [29].
Test drug Jasada Bhasma administered at five times of therapeutic dose significantly elevated serum potassium level in comparison to the normal control group. Although, the precise reason for the elevated serum protein level is unknown, it can be assumed to be due to Bhasma (test drug) pursue itself as it is an inorganic substance. Since, it is observed in higher dose level, it may not be of much relevant at therapeutic doses used in clinical settings. However, it is prudent to monitor electrolyte level when Jasada Bhasma used in the clinical settings at higher dose level over the prolonged period.
In the present study, the test drug Jasada Bhasma administered in therapeutic and five times of therapeutic dose did not elevate the liver enzymes when compared to the control group. It indicates that the test drug does not have marked effect on liver functioning.
Mild histopathological changes in the liver tissue exposed to Jasada Bhasma administered at both the dose levels exhibiting mild sinusoidal dilation and moderate diffused micro fatty changes are indicating relatively good toleration of the drug by the liver which in turn can be correlated with the data of biochemical parameters.
Tissue of kidneies which are exposed to test drug at both levels were showing remarkable pathological changes in their cytoarchitecture which indicates the repeated exposure of Jasada Bhasma endangers the renal function. It is also evident in the significant rise of serum creatinine at both dose levels. This may be the reason for the observed elevation in serum creatinine level.
Similarly, Jasada Bhasma therapeutic dose administered spleen sections showing normal cytoarchitecture but at five times of therapeutic dose, displaying mild to moderate increase in the white pulp proportion can be explained under the light of haemo-lymphatic disturbances upon exposure to a higher concentration of the Bhasma than the normal.
Bone marrow sections of Jasada Bhasma administered at the therapeutic dose and five times of therapeutic dose group has shown overall cellularity is moderate to less in comparison to normal control groups. No cells with micronuclei could be found. Few reticulocytes were also seen. Eosinophilic cells were observed in slightly increased number. The cells from myelomonocytic lineage were more in contrast to erythroid lineage. Megakaryocytes were present in normal number.
Limitation
Detailed characterisation of the preparation using analytical and imaging techniques to determine the physical and chemical nature of the Bhasma and the possibilities of contamination of heavy metals could not be carried out. Histopathological evaluation of toxicity of Jasada Bhasma on reproductive system is warranted.
Conclusion
Repeated administration of Jasada Bhasma in both therapeutic and five times therapeutic dose level did not produce marked toxic effect. However, the higher dose of the element causing certain physiological changes in kidney resulting in renal pathology cannot be ruled out.
Declaration
The author declares that the normal control group images are used common for two test drugs by the same author and the same images are already published for different test drug.
{Data expressed in Mean±SEM, *p<0.05, **<0.01 in comparison to normal control- ANOVA
Hb: Haemoglobin; WBC: White blood cells; RBC: Red blood cells; PCV: Packed cell volume; MCV: Mean corpuscular volume, MCH: Mean corpuscular haemoglobin; MCHC: Mean corpuscular haemoglobin concentration; RDWCV: Red cell distribution width-coefficient of variation; RDWSD: Red cell distribution width-standard deviation; PLT: Platelet count}
{Data expressed in Mean±SEM, *p<0.05, **<0.01 in comparison to normal control-ANOVA
SGOT: Serum glutamic oxaloacetic transaminase; SGPT: Serum glutamic pyruvic transaminase; ALP: Alkaline phosphatase}