Hyponatremia Induced by Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers-A Pilot Study
S Bhuvaneshwari1, Prakash Vel Sankhya Saroj2, D Vijaya3, M Sabari Sowmya4, R Senthil Kumar5
1 Professor, Department of Pharmacology, PSG Institute of Medical Sciences and Research, Coimbatore, Tamil Nadu, India.
2 Undergraduate Student, Department of Pharmacology, PSG Institute of Medical Sciences and Research, Coimbatore, Tamil Nadu, India.
3 Professor, Department of Biochemistry, PSG Institute of Medical Sciences and Research, Coimbatore, Tamil Nadu, India.
4 Undergraduate Student, Department of Pharmacology, PSG Institute of Medical Sciences and Research, Coimbatore, Tamil Nadu, India.
5 Assistant Professor, Department of Endocrinology, PSG Institute of Medical Sciences and Research, Coimbatore, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. S Bhuvaneshwari, Professor, Department of Pharmacology, PSG Institute of Medical Sciences and Research, Coimbatore, Tamil Nadu, India.
E-mail: bhuvana1421@gmail.com
Introduction
Hyponatremia, serum sodium <135 mmol/L, can result in neurological manifestation in acute cases, may lead to seizures and coma. Angiotensin Converting Enzyme Inhibitor (ACEI) and Angiotensin II Receptor Blockers (ARB) are drugs that have been commonly prescribed for the treatment of hypertension and cardiac diseases. It has become important to evaluate and investigate the incidence of hyponatremia on consumption of these drugs.
Aim
To determine the susceptibility of patients on ACEI and ARB to hyponatremia and to ascertain the drug producing notable hyponatremia among ACEI and ARB.
Materials and Methods
The study was conducted in a tertiary care hospital. Serum sodium levels were assayed in patients taking ACEI and ARB; 50 patients were recruited. The patient’s age, sex, drug dosage, frequency of the drug administration were collected using a proforma. Statistical analysis of data were performed using SPSS version 19.0.
Results
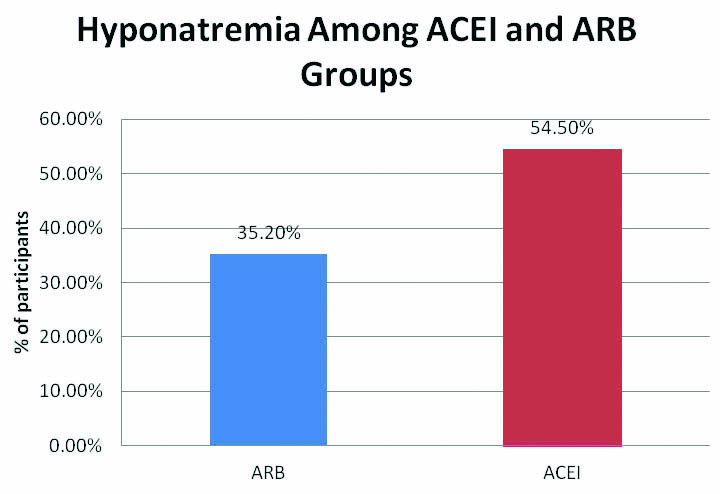
Among all, 48% (24 out of 50) of the study population administered with ACEI and ARB developed hyponatremia. Predisposition to develop hyponatremia was high in males compared to females. Incidence of hyponatremia was 62.5% (10 out of 16) in the age group of 56-75 years. Though, incidence of hyponatremia was 54.5% (18 out of 33) in ACEI group compared to 35.2% (6 out of 17) in ARB group, but it was not statistically significant. The study also revealed that metosartan had a higher association with hyponatremia compared to other drugs.
Conclusion
Hyponatremia was induced in nearly 50% of patients taking ACEI and ARB. The incidence of hyponatremia among patients on these two drugs did not show statistical variation. Metosartan showed a higher incidence of hyponatremia compared to enalapril, ramipril, captopril, telmisartan, losartan included in the study. This study revealed that monitoring of serum sodium level in the patients with ACEI and ARB administration will help to elude unexpected adverse reactions.
Angiotensin converting enzyme inhibitors, Angiotensin II receptor blockers, Lowering of sodium levels
Introduction
Hyponatremia is the most common electrolyte abnormality in hospitalised patients. Hyponatremia may be associated with disease conditions or as an adverse effect of certain drugs. Medicines have been reported to account for 30% cases of hyponatraemia [1]. Hyponatremia may develop occasionally in the course of treatment with drugs used in every day clinical practice. ACEI and ARB, known as AT1 receptor antagonists or Sartans, modulate the Renin Angiotensin Aldosterone system, are drugs primarily used for the treatment of hypertension, diabetic nephropathy and congestive heart failure. The rate of prescribing ACE Inhibitors is around 13.5% and AT1 receptor antagonists are 24% in major cities of India [2]. The commonly prescribed drugs include ‘Enalapril’, ‘Ramipril’ and ‘Lisinopril’ (ARB) and losartan, candesartan, irbesartan (ACEI). The drug reactions reported include hypotension, cough, hyperkalemia, headache, dizziness, nausea and renal impairment. A case report on monitoring of serum sodium levels before and after therapy by Izzedine H et al., reported severe hyponatremia by enalapril which was ascribed to ACEI-induced Syndrome of Inappropriate Antidiuretic Hormone (SIADH) [3]. Reports also indicate effects of ACEI and ARB on sodium channels: ACEI-treatment alters sodium transporter sub cellular distribution in a manner that would blunt sodium reabsorption along the nephron [4]; ACEI and ARB’s have also been reported to down regulate the sodium channel and renin expression in renal tubules. These effects would act to suppress sodium reabsorption via sodium channel and neutralises the mechanism that would elevate blood pressure in response to increased salt intake [5]. ARB has been reported to inhibit the vaso-constricting mechanism and aldosterone secreting effects of angiotensin II leading to decreased renal tubular sodium reabsorption and potassium secretion [6].
Clinical manifestations of hyponatremia are primarily neurologic (due to an osmotic shift of water into brain cells causing oedema), especially in acute hyponatremia, and include dizziness, headache and hyperkalemia. Infrequent side effects include orthostatic hypotension, rash, diarrhoea, dyspepsia, abnormal liver function, muscle cramp, myalgia, nasal congestion and back pain. Chronic hyponatremia (>48 hour) patients may appear asymptomatic. It is associated with multiple poor clinical outcomes including falls, fractures, increased length of hospital stay and mortality [7]. Physicians may not always provide proper attention on time to undesirable drug-induced hyponatremia. Awareness of this less common adverse effect of these drugs on serum sodium levels is of great importance for prevention and prompt and effective clinical management. Therefore, the present study was conducted to determine the susceptibility of patients on ACEI and ARB to hyponatremia and to ascertain the drug producing notable hyponatremia among ACEI and ARB.
Materials and Methods
A cross-sectional study was conducted on the patients visited the tertiary care hospital at Tamil Nadu, India, from August 2015 to December 2015 after approval from Institutional Human Ethics Committee. Being a pilot study 50 patients was recruited to evaluate the prevalence of hyponatremia induced by ACEI and ARB.
Patient’s above18 years, both sex, having ACEI or ARB for minimum of one month were included in the study. Patients with known renal failure, history of diarrhoea during past one week and intake of additional drugs that have been known to cause hyponatremia were excluded from the study.
Written informed consent was obtained from eligible patients based on inclusion and exclusion criteria. A 3 mL of venous blood were collected in sterile conditions for assay of serum sodium level. The patient’s age, sex, drug dosage, frequency of the drug administration were collected using proforma and serum sodium levels were assayed by direct ISE method.
Statistical Analysis
Statistical analysis of data were performed using SPSS version 19.0. Chi-square test was used to compare occurrence of hyponatremia among patients taking ACEI and ARB. Patients were grouped based on their sodium levels as normal and hyponatremic, their mean sodium levels were compared using Student’s t-test. The hyponatremic group was further sub-divided into ACEI and ARB groups to compare their mean sodium levels using Student’s t-test. p<0.5 was considered as statistically significant.
Results
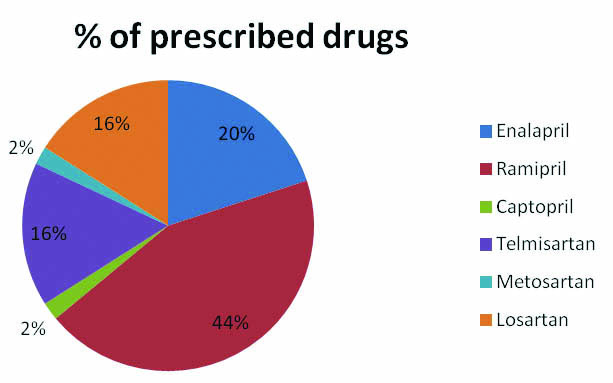
A total of 50 participants recruited for the study, 33 on ACEI and 17 on ARB. Approximately 48% (24 out of 50) of patients administered with ACEI and ARB developed hyponatremia. Predisposition to develop hyponatremia was found to be more in males compared to females, 51% of males developed hyponatremia compared to 38% of females. Mean sodium levels were also lower in males compared to females [Table/Fig-1]. Incidence of hypoanatremia was more (62.5%) in the older age group (56-75 years) compared to the 45-55 years age group. However, the magnitude of lowering of sodium level was higher in patients above 66 years indicating a higher drop in serum sodium level as age advances [Table/Fig-1]. ACEI had a higher rate of inducing hyponatremia compared to ARB [Table/Fig-2]. The study population was prescribed Enalapril, Ramipril, Captopril, Telmisartan, Metosartan or Losartan. Among these Ramipril was the commonly prescribed drug and Metosartan and Captopril were the less prescribed [Table/Fig-3]. However, the occurrence of hyponatremia was more with Metosartan compared to other drugs [Table/Fig-4].
Incidence of hyponatremia with ACEI and ARBs.
| Total number of participants | Participants with hyponatremia |
|---|
| n (%) | Mean sodium level (meq/L) | n (%) | Mean sodium level (meq/L) |
|---|
| Total | 50 (100) | 135.0 | 24 (48) | 131.1 |
| Male | 37 (74) | 134.6 | 19 (51) | 130.7 |
| Female | 13 (26) | 136.4 | 5 (38) | 132.3 |
| 45-55 years | 20 (40) | 136.1 | 7 (36.8) | 131.6 |
| 56-65 years | 15 (30) | 133.46 | 10 (62.5) | 131.2 |
| >66 years | 15 (30) | 135.13 | 7 (50) | 130.4 |
Incidence of hyponatremia among ACEI and ARB.
Percentage of prescribed drugs.
Hyponatremia induced by individual drugs.
| Drugs | Total Number of Participants | Participants with Hyponatremia n (%) |
|---|
| ARB | Telmisartan | 8 | - |
| Metosartan | 1 | 1 (100) |
| Losartan | 8 | 1 (12.5) |
| ACEI | Enalapril | 10 | 6 (60) |
| Ramipril | 22 | 12 (54.5) |
| Captopril | 1 | 0 (0) |
ARB: Angiotensin II receptor blockers; ACEI: Angiotensin converting enzyme inhibitor
Discussion
Hyponatremia may occur due to excess amount fluid compared to normal level of sodium or due to depletion of both sodium and body fluid. The symptoms include nausea, malaise, and lethargy, decreased level of consciousness, headache, seizures and coma. When there is very low serum sodium levels (<115 meq/L), it can even lead to intracerebral osmotic fluid shifts and brain oedema which may become fatal.
Medical conditions that can sometimes be associated with hyponatremia are adrenal insufficiency, hypothyroidism, and cirrhosis of the liver. Additionally administration of certain drugs like diuretics, vasopressin, and the sulfonylurea drugs has been reported to lower serum sodium levels. It is often considered that there is no effect of ACEI and ARB on serum sodium levels. However, in the recent past years there are few case reports indicate incidence of hyponatremia due to ACEI and ARB administration [3,8,9].
The present study revealed the occurrence of hyponatremia in 48% of the participants on ACEI and ARB [Table/Fig-1]. This finding coincides with more incidence of hyponatremia with the case reports on ACEI induced hyponatremia [3,8,9].
A previous study on losartan had reported higher susceptibility of females to hyponatremia compared to men [10]. However, the present study results indicate higher susceptibility of males compared to females; however the difference was not statistically significant [Table/Fig-1]. This may also be due to the fact that cardiovascular and hypertensive diseases are more predominant in males than in females and thus the proportion of male population receiving the drugs are increased in number.
Incidence of hyponatremia was high in the age group 56-75 years compared to the lower age groups (44-55 years). However, the magnitude of serum sodium lowering was found to be higher in patients above 66 years of age [Table/Fig-1]. A study on patients receiving losartan had also reported the occurrence of hyponatremia at a mean age of 76.4 years [10]. Variations in the kinetics and dynamics of drugs in elderly patients could be basis for this finding.
The mean sodium levels were lower (130.8 meq/L) in ACEI group than in ARB group (131.8 meq/L); though, the incidence of patients who developed hyponatremia was higher in ACEI group than ARB group, it was not statistically significant (p>0.05) [Table/Fig-2]. Also, literature search on earlier case reports revealed that ACEI induced hyponatremia had been reported more frequently than on ARB usage [2,8-12]. Even though both ACEI and ARB induces hyponatremia, the incidence was more with ACEI because of increase in Angiotensin I levels with ACEI.
The drugs prescribed for the present study group patients include enalapril, ramipril, captopril, telmisartan, metosartan and losartan [Table/Fig-3]. The incidence of hyponatremia was found to be higher with metosartan. Only one patient (2%) of the study group was prescribed captopril but no hyponatremia was reported in this patient [Table/Fig-4]; though, earlier case reports on enalapril, lisinopril, captopril, ramipril and losartan have reported hyponatremia [3,8-14].
As 48% of the study group developed hyponatremia, the results of present study highlight the need for monitoring serum sodium levels in patients with ACEI and ARB administration.
The mechanism of ACE inhibitor and ARB induced hyponatremia has not been confirmed. Syndrome of inappropriate secretion of antidiuretic hormone associated with ACE inhibitor and ARB therapy inducing symptomatic hyponatremia could be considered as a rare but possible cause [3,12]. With ACEI therapy increased circulating Angiotensin I enters the brain and is converted to Angiotensin II, which may stimulate thirst and release of antidiuretic hormone from the hypothalamus, eventually leading to hyponatremia [8]. Reports also indicate there is blockade of Aldosterone induced renal tubular sodium reabsorption and potassium secretion which might lead to lowering of serum sodium levels [6].
Limitation
The susceptibility of individuals to develop hyponatremia and the type of drug involved in producing hyponatremia may have genetic disposition. This could be the cause for the diversity observed in the present and previous studies. Further exploration on this aspect requires studies with larger sample size to through more light on hyponatremia induced by these drugs.
Conclusion
Hyponatremia was found to be associated in nearly 50% of patients taking AECI and ARB. The incidence of hyponatremia among patients on these two drugs did not show statistical variation. Though, metosartan showed a higher incidence of hyponatremia compared to other drugs included in the study, studies with larger sample size are required to confirm the results. Present study emphasises the need for monitoring of serum sodium level in patients on ACEI and ARB administration to elude unexpected adverse reactions.
ARB: Angiotensin II receptor blockers; ACEI: Angiotensin converting enzyme inhibitor
[1]. Yawar A, Jabbar A, Haque NU, Zuberi LM, Islam N, Akhtar J, Hyponatraemia: etiology, management and outcomeJournal of the College of Physicians and Surgeons Pakistan 2008 18(8):467-71. [Google Scholar]
[2]. Dahake P, Banerjee SK, Market investigation study of angiotensin converting Enzyme (ACE) inhibitors in a major Urban City of IndiaApeejay-Journal of Management Sciences and Technology 2017 4(2):19-28. [Google Scholar]
[3]. Izzedine H, Fardet L, LaunayVacher V, Dorent R, Petitclerc T, Deray G, Angiotensin converting enzyme inhibitor induced syndrome of inappropriate secretion of antidiuretic hormone: Case report and review of the literatureClinical Pharmacology & Therapeutics 2002 71(6):503-07.10.1067/mcp.2002.12452012087354 [Google Scholar] [CrossRef] [PubMed]
[4]. Moritz ML, Ayus JC, The pathophysiology and treatment of hyponatraemic encephalopathy: an updateNephrology Dialysis Transplantation 2003 18(12):2486-91.10.1093/ndt/gfg39414605269 [Google Scholar] [CrossRef] [PubMed]
[5]. Ushio-Yamana H, Minegishi S, Ishigami T, Araki N, Umemura M, Tamura K, Renin angiotensin antagonists normalize aberrant activation of epithelial sodium channels in sodium-sensitive hypertensionNephron Experimental Nephrology 2012 122(3-4):95-102.10.1159/00034866023594971 [Google Scholar] [CrossRef] [PubMed]
[6]. Cakir M, Significant hyperkalemia and hyponatremia secondary to telmisartan/hydrochlorothiazide treatmentBlood pressure 2010 19(6):380-82.10.3109/08037051.2010.48805620486869 [Google Scholar] [CrossRef] [PubMed]
[7]. Soiza RL, Hoyle GE, Chua MP, Electrolyte and salt disturbances in older people: causes, management and implicationsReviews in Clinical Gerontology 2008 18(2):143-58.10.1017/S0959259809002822 [Google Scholar] [CrossRef]
[8]. Shaikh ZH, Taylor HC, Maroo PV, Llerena LA, Syndrome of inappropriate antidiuretic hormone secretion associated with lisinoprilAnnals of Pharmacotherapy 2000 34(2):176-79.10.1345/aph.1911610676825 [Google Scholar] [CrossRef] [PubMed]
[9]. Al-Mufti HI, Arieff AI, Captopril-induced hyponatremia with irreversible neurologic damageThe American Journal of Medicine 1985 79(6):769-71.10.1016/0002-9343(85)90530-3 [Google Scholar] [CrossRef]
[10]. Kinoshita H, Kobayashi K, Yaguramaki T, Yasuda M, Fujiki K, Tomiyama J, Losartan potassium/hydrochlorothiazide (Preminent®) and hyponatremia: Case series of 40 patientsHuman & experimental toxicology 2011 30(9):1409-14.10.1177/096032711038745520974655 [Google Scholar] [CrossRef] [PubMed]
[11]. Tilly Gentric A, Severe hyponatremia associated with ramipril therapy in an old womanJournal of the American Geriatrics Society 1995 43(12):1448-79.10.1111/j.1532-5415.1995.tb06638.x7490409 [Google Scholar] [CrossRef] [PubMed]
[12]. Castrillon JL, Mediavilla A, Mendez MA, Cavada E, Carrascosa M, Valle R, Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH) and enalaprilJournal of Internal Medicine 1993 233(1):89-91.10.1111/j.1365-2796.1993.tb00655.x8429295 [Google Scholar] [CrossRef] [PubMed]
[13]. Chakithandy S, Evans R, Vyakarnam P, Acute severe hyponatraemia and seizures associated with postoperative enalapril administrationAnaesth Intensive Care 2009 37(4):673-74. [Google Scholar]
[14]. Goto Y, Wakita S, Yoshimitsu M, Inagaki S, Kobayashi T, Kaneko S, Onset of syndrome of inappropriate secretion of antidiuretic hormone in a gastric cancer patient on SOX treatmentGan To Kagaku Ryoho 2015 42(13):2467-70. [Google Scholar]