Sexually transmitted infections, caused by TV, CT, and NG, Treponema pallidum (syphilis), and hepatitis B are common in pregnancy [1-4]. STIs are associated with adverse birth outcomes and increased risk of HIV acquisition [3-8]. While these infections may cause morbidity and mortality, any STI, if identified properly, is curable or manageable.
India, one of the most populous countries in the world, continues to struggle to provide equitable quality healthcare to its citizens with clear disparities amongst women and those living in rural areas [9]. A 2011 report based on the Indian census from 2010 found there were over 400 million women living in rural areas [10]. Another study from 2010, estimated about 12.8 million neonates in India were born underweight for their gestational age-nearly half of all births [11]. Despite known associations between STIs and adverse birth outcomes, STIs are addressed using syndromic management in India, partially due to lack of laboratory facilities needed for diagnostic testing [12]. Syndromic management is inadequate for STI control because it not only leads to overtreatment due to the non-specificity of symptoms, but also under diagnosis and missed asymptomatic infections [13,14]. With high rates of adverse birth outcomes, poor surveillance data on STIs, and known associations between STIs and adverse birth outcomes, the present study was carried out to investigate the relationship of select STIs and adverse birth outcomes using stored, frozen vaginal swabs from a cohort of pregnant women living in Southern India.
Materials and Methods
A prospective cohort study was conducted by the Public Health Research Institute of India, between May 2011 and June 2014 that provided integrated antenatal care and HIV testing services to 1,906 pregnant women living in rural communities in Mysore district, India. The study has been described in detail elsewhere [15]. The study was reviewed and approved by the Institutional Review Board at Public Health Research Institute of India, Mysore, India (Protocol number 2011-03-26-10). All participants underwent informed consent process before participating in the program. They were informed of study procedures and their ability to withdraw from the study at any time.
In brief, pregnant women willing to attend all program visits undergo antenatal care consultation and HIV testing at mobile medical clinics, and undergoing informed consent process were included in the program. Interviewer-administered surveys collected detailed information on sociodemographic, medical, and obstetric history in the local language of Kannada. A nurse phlebotomist collected sera samples from all participants. Vaginal samples were self-collected from all women attending the clinic. Postnatal follow-up surveys were conducted within 15 days, six months, and one year of the date of delivery.
All pregnant women were screened for signs and symptoms of STIs. Questions that were asked to elicit information about symptoms of STIs included: In the last three months did you notice any pain or burning during urination, non-traumatic sores or boils, ulcers, itching, vaginal discharge, or lower abdominal pain. Women with one or more symptoms were categorised as symptomatic. Women were also asked treatment history to assess if they were potentially treated for any STIs.
All laboratory investigations were conducted shortly after specimen collection. Sera samples were tested for HIV antibodies {ErbaLisa HIV 1+2 Gen3 (Erba Mannheim, Mannheim, Germany) and HIV TRI-DOT (J. Mitra & Co. Pvt. Ltd., New Delhi, India)}, hepatitis B antibodies {ErbaLisa Hepatitis B surface antibody test (Erba Mannheim, Mannheim, Germany)}, and syphilis antibodies with Venereal Disease Research Laboratory (VDRL) test {Rapid Plasma Reagin (RPR) (Span Diagnostics, Surat, India)}. All pregnant women diagnosed with these infections were either treated or referred to a tertiary care centers for further management.
Vaginal swab samples were stored at -80°C and later used in the present study. Pregnant women were selected if they had experienced any adverse birth and infant outcomes, which were defined as newborns that weighed less than 2.5 kilograms, delivered before 37 weeks of gestation, or were stillborn, died, or admitted to an intensive care unit at birth. Pregnant women who delivered a full-term baby with normal birthweight were compared to the aforementioned women with similar age and parity.
Stored vaginal swabs were thawed and tested for TV, CT, and NG with real-time PCR (Xpert® TV, Xpert® CT/NG; Cepheid, Sunnyvale, CA) at the Postgraduate Institute of Medical Education and Research in Chandigarh, India using the GeneXpert® system. In-house samples of TV, CT, and NG were used as quality controls.
A cycle threshold value is the number of cycles a polymerase chain reaction undergoes to reliably detect a gene of interest. Cycle threshold values are used in real time nucleic acid amplification tests to determine whether a test is interpreted as positive or negative. A lower cycle threshold values indicate that a tested sample has a larger amount of initial genetic material [16]. Among pregnant women that tested positive for any STI, we looked for differences in mean cycle threshold values between symptomatic and asymptomatic women, to assess if there were any differences in the mean biological burden of infection.
Statistical Analysis
All descriptive statistics and prevalence ratios with associated 95% confidence intervals (95% CI) were calculated using Stata SE 14.1 (College Station, TX). The present study compared pregnant women with and without adverse birth outcomes in a one-to-one ratio, and we were powered to detect a difference in prevalence ratio of 2.00. A prevalence ratio was used for this analysis versus an odds ratio because it is a better descriptor of different states between those groups [17]. Student’s t-test was used to compare cycle threshold values of symptomatic and asymptomatic women that tested positive for TV infection.
Results
The present study compared 208 women with adverse birth outcomes to 213 women that had normal birth outcomes. Valid test results were obtained for 407 (95%) samples tested for TV, with 18 invalid tests and three test errors. Valid test results were achieved for 421 (98%) samples tested for CT/NG, with five invalid tests, one test error, and one test with no result. The duration of storage of vaginal swab specimens for women with and without adverse birth outcomes was similar (19 to 30 months).
The median age of women with an adverse birth outcome was 21-year-old {Interquartile range (IQR): 19-23} and the median age of women without an adverse birth outcome was 21 years old (IQR: 19-22), on the date of specimen collection about 45% of the women reported no prior pregnancy in both groups. TV was the most common infection among women in both groups [Table/Fig-1]. In the present study, we found that women with adverse birth outcomes (10.6%) had a higher ratio of STI than those without adverse birth outcomes {6.6%; prevalence ratio of 1.7, 95% (CI: 0.9-3.3); [Table/Fig-1]}. In-house samples of TV, CT, and NG, were frozen, stored, and thawed for similar periods of time and were used as positive controls for Xpert® real-time testing and were all found to test positive.
Sociodemographic characteristics and sexually transmitted infections among pregnant women with and without adverse birth outcomes* in rural India, 2013-2014 (n=421).
| Women with adverse birth outcomes* | Women with normal birth outcomes |
|---|
| (n=208) | % | (n=213) | % |
|---|
| Maternal median age (years), interquartile range | 21 | (19, 23) | 21 | (19, 22) |
| Completed education** |
| None | 13 | 6.3% | 11 | 5.2% |
| Primary | 80 | 38.0% | 64 | 30.0% |
| Middle school | 71 | 34.0% | 96 | 45.0% |
| Secondary or above | 44 | 21.0% | 42 | 20.0% |
| Primigravida | 93 | 45.0% | 97 | 45.0% |
| Biological Tests |
| Positive TV Test*** | 15 | 7.2% | 12 | 5.6% |
| Positive CT Test | 1 | 0.5% | 0 | 0.0% |
| Positive NG Test | 1 | 0.5% | 1 | 0.5% |
| Positive TV, CT, and NG Test | 0 | 0.0% | 1 | 0.5% |
| Positive HIV Elisa | 2 | 1.0% | 0 | 0.0% |
| Positive HBsAg Serology | 3 | 1.4% | 0 | 0.0% |
| Positive VDRL test | 0 | 0.0% | 0 | 0.0% |
| Any STI | 22 | 10.6% | 14 | 6.6% |
Trichomonas vaginalis (TV); Chlamydia trachomatis (CT); Neisseria gonorrhoeae (NG); Human immunodeficiency virus (HIV); Hepatitis B surface antigen (HBsAg); Venereal disease research laboratory (VDRL); Sexually transmitted infection (STI)
*Adverse birth outcomes defined as: newborns that weighed less than 2.5 kg (n=91), died (n=50), delivered before 37 weeks of pregnancy (n=26), or admitted to an intensive care unit at birth (n=41).
**Education: Primary= 1-8 years; middle school= 9-10 years; secondary or above= 11+ years.
***n=407
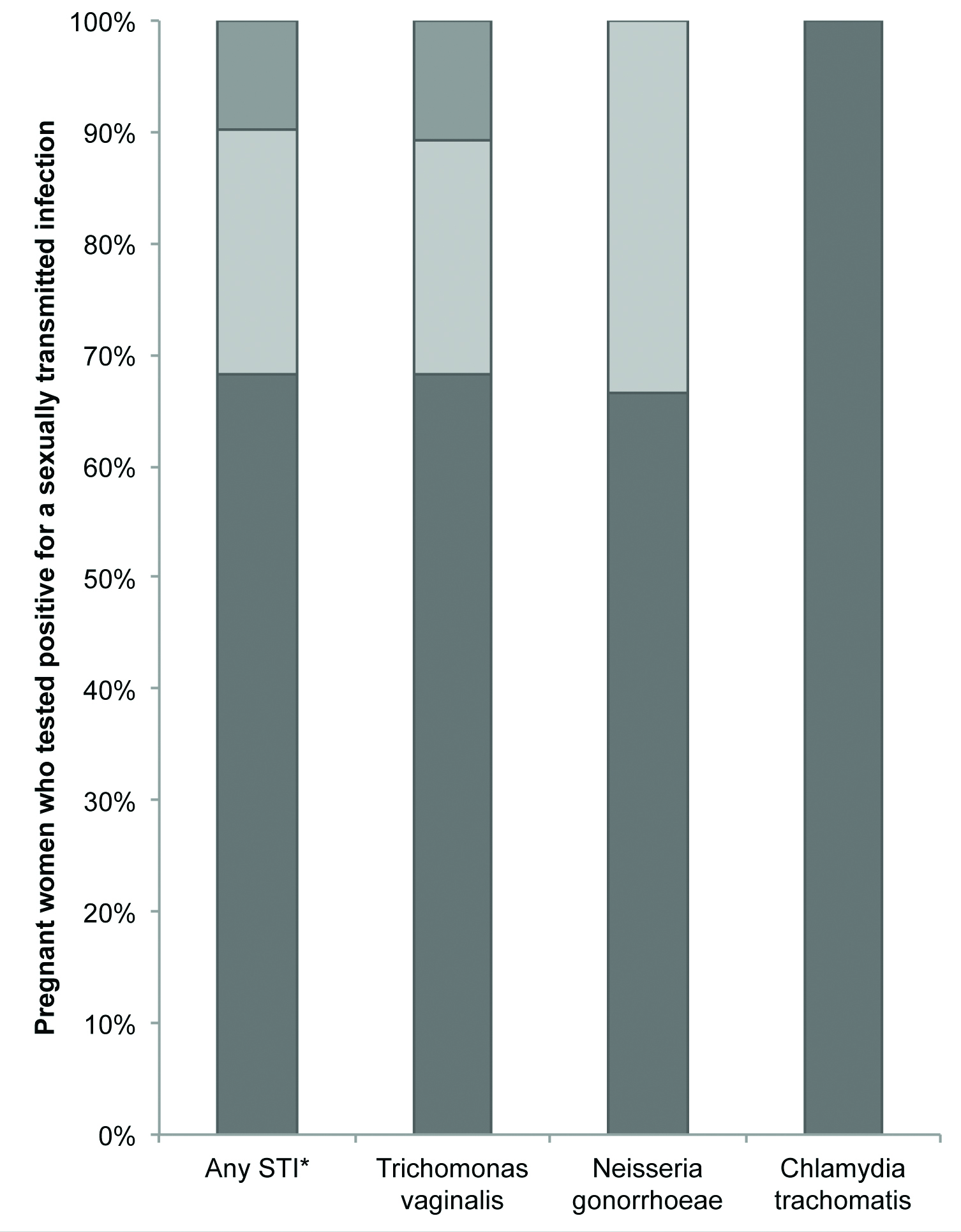
Of the 28 women who tested positive for TV, 67.7% were asymptomatic. Of the two women that tested positive for CT, both (100%) were asymptomatic. Of the three women that tested positive for NG, 2 (67.67%) were asymptomatic. In total, 68% of women who tested positive for TV, CT, or NG were asymptomatic. Three of the 9 (32.33%) symptomatic women that tested positive for TV were treated using the Indian STI management strategy. Among women with any TV, CT, or NG infection, 9.7% of women had been treated [Table/Fig-2].
Trichomonas vaginalis, Neisseria gonorrhoeae, andChlamydia trachomatis infections that were symptomatic, asymptomatic, and treated among pregnant women.
 |
|---|
| Asymptomatic | Symptomatic** | Treated |
|---|
| Any sexually transmitted infection* | 67.7% | 32.3% | 9.7% |
| Trichomonas vaginalis | 61.3% | 29.0% | 9.7% |
| Neisseria gonorrhoeae | 6.5% | 3.2% | 0.0% |
| Chlamydia trachomatis | 6.5% | 0.0% | 0.0% |
*One asymptomatic pregnant woman was co-infected with Trichomonas vaginalis, Neisseria gonorrhoeae, and Chlamydia trachomatis
**Only pregnant women with symptomatic infections reported treatment
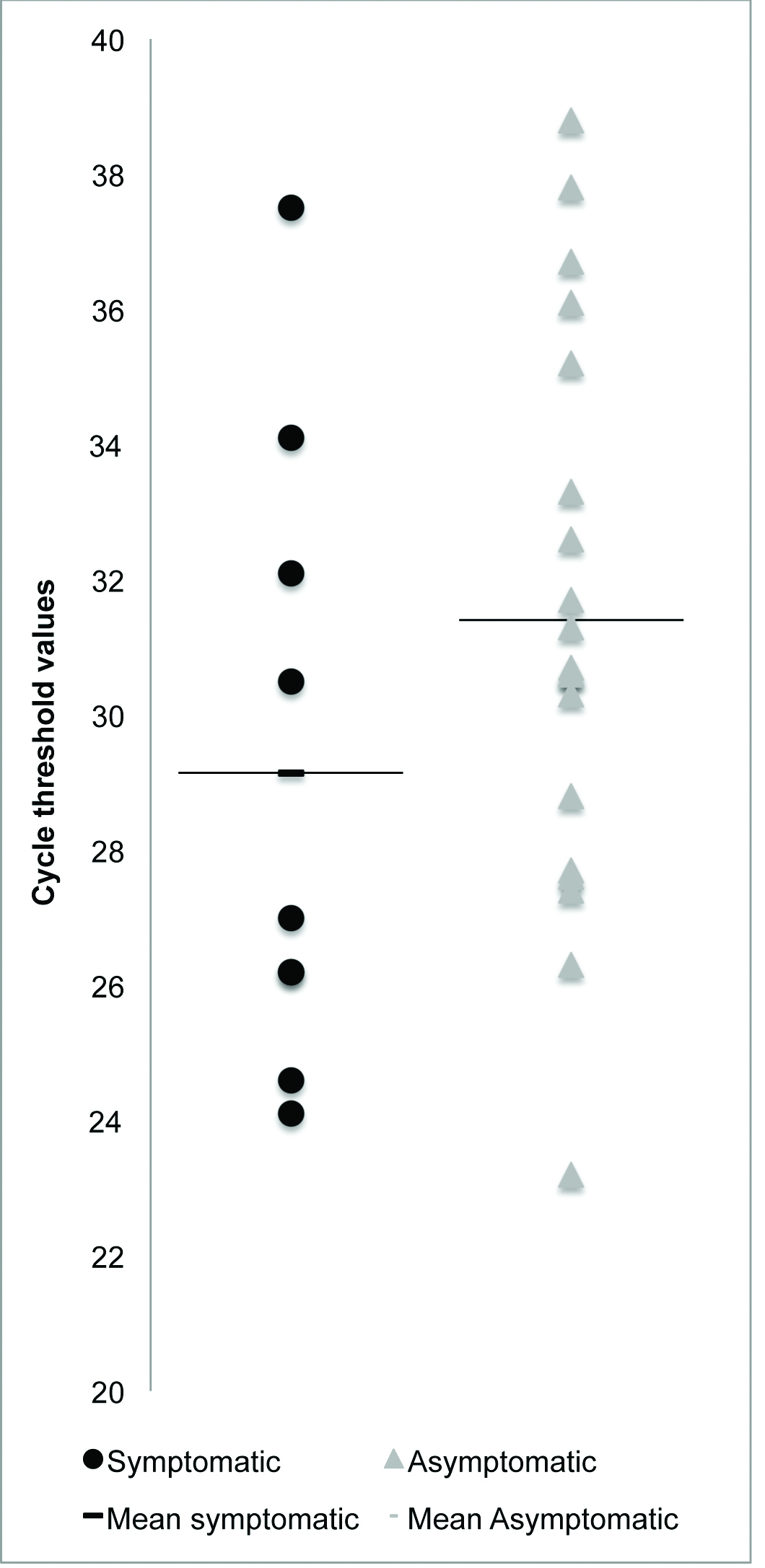
Among pregnant women that tested positive for TV infection, there was no difference between mean cycle threshold values between symptomatic (mean cycle threshold value: 27) and asymptomatic (mean cycle threshold value: 30.7) women (p-value: 0.104 {standard error 0.827} [Table/Fig-3].
Cycle threshold values of polymerase chain reaction tests for the detection of a specific Trichomonas vaginalis deoxyribonucleic acid sequence among symptomatic and asymptomatic pregnant women.
Discussion
In the present study, we found that pregnant women with adverse birth outcomes had a higher ratio of STI than those without adverse birth outcomes. Present findings are in agreement with prior studies that observed that adverse birth outcomes were associated with STIs [3,4,6-8]. Among tested pregnant women, a modest number of STIs were detected in rural Mysore using highly sensitive and specific nucleic acid amplification testing [18,19]. Many women in the present study had asymptomatic STI infections and had not been treated for their infections. Although, we did not observe a significant difference between cycle threshold values, a measure of organism burden, between symptomatic women with TV compared to asymptomatic women, there was a trend, indicating that asymptomatic women may have lower burden of TV organisms. Cycle threshold values among asymptomatic and symptomatic TV infections have not been described before in the literature on STIs in pregnant women in India.
We used specimens stored at -80°C that were collected as far as two and a half years prior to the date of the nucleic acid amplification tests, which is beyond the length of recommended clinical testing. Prior studies have found that CT DNA was still detectable by PCR amplification for upto two years after sample collection [20]. Additionally, Xpert® cartridges have an internal sample control that amplifies a ubiquitous single human gene to ensure that participant samples have been properly collected, appropriate testing conditions have occurred, and amplifiable DNA was still present [16]. Almost all of the present samples had valid results on the Xpert® system and replicable results with confirmed stored sample controls for TV, CT, and NG. The present study showed that TV, CT, and NG testing is feasible and reliable up to two years after vaginal sample collection when stored at -80°C.
The present study also had several strengths. We used highly sensitive real-time PCR testing to detect STIs, which does not require viable organisms to be present in the sample, improving STI detection [20]. All laboratory investigations were done using standardised protocols by trained personnel for STI diagnosis.
In addition, due to limited STI surveillance data, those data could be useful for public health officials in the allocation and management strategies of STIs among pregnant women residing in rural India. A prior study found a similar prevalence of TV infection (8.5%) among non-pregnant women in Mysore district, India [21]. A study from 2009 found that among non-pregnant women, 41% of the culture-positive TV infections were asymptomatic [22]. A systematic literature review of CT infection, detected by PCR tests, reported that among pregnant women the prevalence of infection was between 0.1% to 2.5%, in India [23]. That prevalence of CT infection among pregnant women was comparable to present cohort. A community-based study conducted in Mysore, India from 2005 to 2006 reported a prevalence of gonorrhea, detected by nucleic acid amplification tests, among rural women to be 0.2% [24]. Among the pregnant women in the present study, we had two cases of NG infection (0.5%), which was comparable to that study.
Limitation
The present study has limited sample size resulted in an underpowered study, where we could not detect a significant effect size smaller than 0.098. The present study results may not be generalisable to other populations as this was an observational study using a non-probability sample.
Conclusion
Among rural pregnant women in Southern India, women with adverse birth outcomes had a higher ratio of STI than those without adverse birth outcomes. Among pregnant women who were found to test positive for TV, CT, or NG with highly sensitive and specific nucleic acid amplification tests, most were asymptomatic and none of the asymptomatic infections were treated under the current Indian STI management strategy. Given present findings, we show that the introduction of nucleic acid amplification tests to detect infections among pregnant women living in rural communities could better identify STIs, and possibly reduce adverse birth outcomes, especially TV in Southern India.
Availability of Data and Materials
Data and materials can be requested from the Public Health Research Institute of India.
Competing Interests
The authors have no competing interests to declare.
Funding
The Kisalaya mobile clinic project was funded by the Elizabeth Glaser Paediatric AIDS Foundation International Leadership Award to Dr. Purnima Madhivanan. The SCIL project was funded by Positive Action for Children Fund. Kojima and Madhivanan’s time was supported by the Fogarty International Center, National Institutes of Health under award number D43 TW009343 and D43 TW010540 respectively, and the University of California Global Health Institute (UCGHI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Trichomonas vaginalis (TV); Chlamydia trachomatis (CT); Neisseria gonorrhoeae (NG); Human immunodeficiency virus (HIV); Hepatitis B surface antigen (HBsAg); Venereal disease research laboratory (VDRL); Sexually transmitted infection (STI)
*Adverse birth outcomes defined as: newborns that weighed less than 2.5 kg (n=91), died (n=50), delivered before 37 weeks of pregnancy (n=26), or admitted to an intensive care unit at birth (n=41).
**Education: Primary= 1-8 years; middle school= 9-10 years; secondary or above= 11+ years.
***n=407
*One asymptomatic pregnant woman was co-infected with Trichomonas vaginalis, Neisseria gonorrhoeae, and Chlamydia trachomatis
**Only pregnant women with symptomatic infections reported treatment