Mixed Mucinous Male Breast Carcinoma: A Rare Histological Variant of a Rare Disease
Badareesh Lakshminarayana1, Ankita Harijee2, Prashanth Shetty3, Rama Rani Krishna Bhat4
1 Associate Professor, Department of Surgery, Kasturba Medical College, Manipal, Karnataka, India.
2 Senior Resident, Department of Surgery, Kasturba Medical College, Manipal, Karnataka, India.
3 Professor, Department of Surgery, Kasturba Medical College, Manipal, Karnataka, India.
4 Senior Resident, Department of Anaesthiology, Kasturba Medical College, Manipal, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Badareesh Lakshminarayana, Associate Professor, Department of Surgery, Kasturba Medical College, Manipal-576104, Karnataka, India.
E-mail: drbadareesh.l@gmail.com
Male breast carcinoma is rare neoplasm, accounting for <1% of all diagnosed breast malignancy. Histologically, most often it is infiltrating ductal carcinoma and rarely mucinous carcinoma. A 73-year-old male presented with lump in right breast since six months. On examination a 5x5 cm hard, non tender lump fixed to overlying skin, with no right axillary lymphadenopathy. Incisional biopsy showed mixed mucinous carcinoma of breast. After investigations he underwent right modified radical mastectomy. Histopathology confirmed mixed mucinous carcinoma and infiltrating ductal carcinoma. Patient received adjuvant radiotherapy, chemotherapy and hormonal therapy. Mixed mucinous and infiltrating ductal carcinoma is extremely rare. Clinical stage and axillary lymph node status are of prognostic value.
Hormonal therapy, Infiltrating duct carcinoma, Modified radical mastectomy
Case Presentation
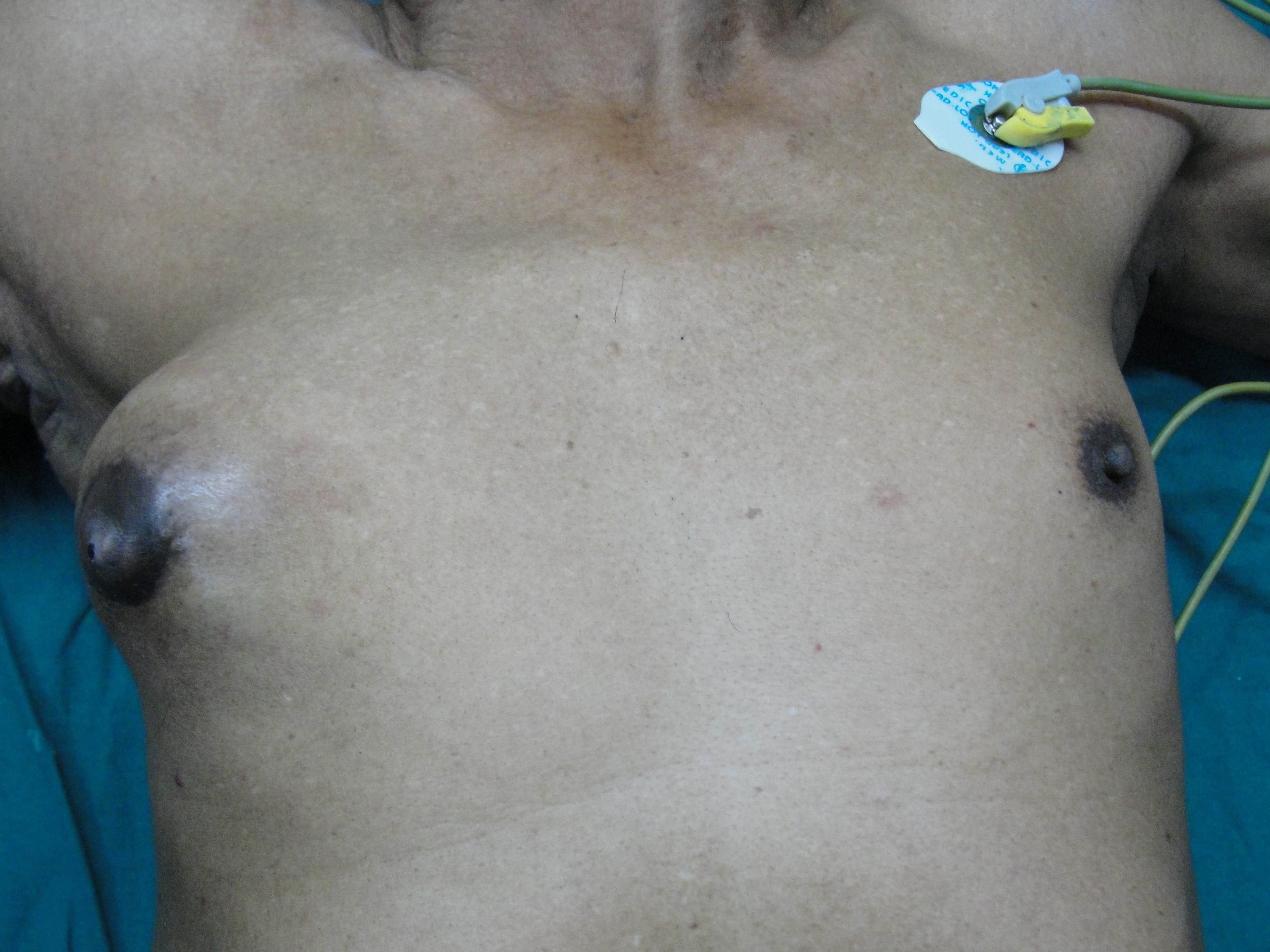
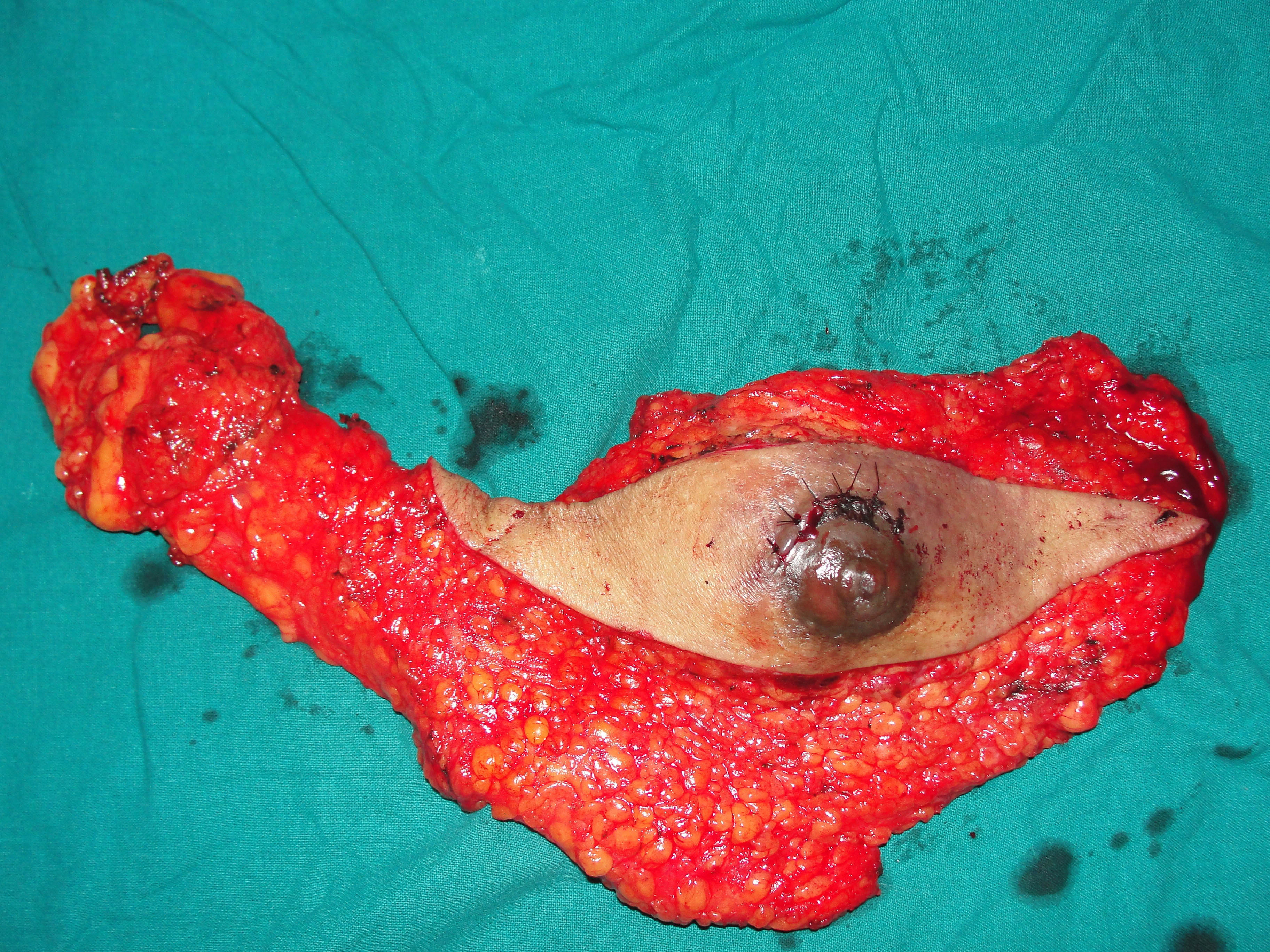
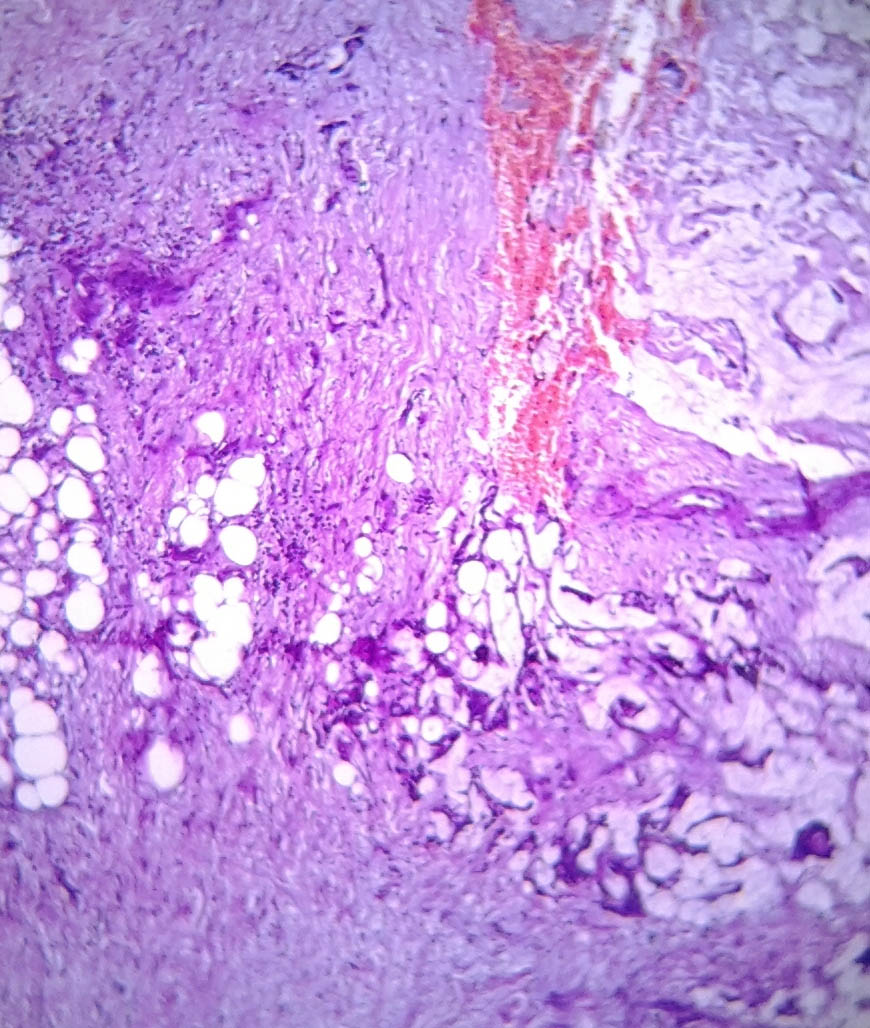
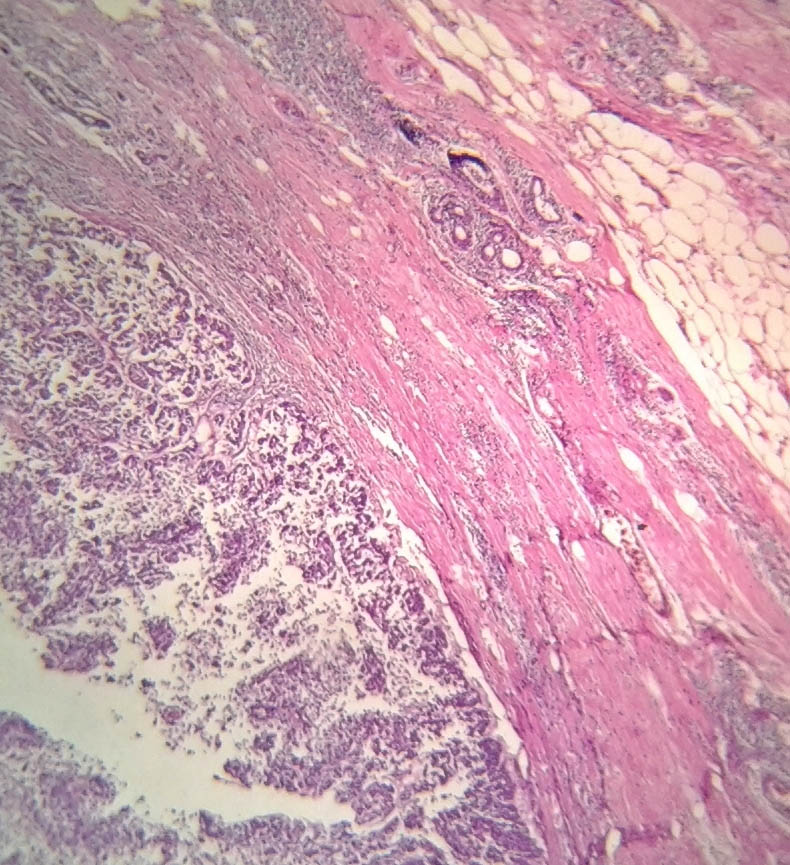
A 73-year-old male presented with a retroareolar hard lump in right breast for the past six months. There was no history of nipple discharge or familial breast cancer. Physical examination revealed a well circumscribed hard, non tender nodule, measuring 5×5 cm in diameter in all the quadrants of right breast, fixed to overlying skin with no skin changes like peau d orange appearance or ulceration. Axillary lymph nodes were not palpable. Contralateral breast was normal on examination [Table/Fig-1]. Provisional diagnosis of carcinoma of right breast was made. Ultrasonography of the breast done at local hospital showed a 7x5.8 cm heterogeneous mass in the right breast. Mammography of the breast and sentinel lymph node biopsy were not done as these facilities were not available and patient’s financial condition was not suitable for further investigations at higher centre. As Fine Needle Aspiration Cytology (FNAC) done twice was inconclusive, an incisional biopsy from the breast lump was done and histopathology was suggestive of mixed mucinous carcinoma of breast with infiltrating ductal carcinoma component. Bone scan and Contrast Enhanced Computerised Tomography (CECT) of chest and abdomen were normal. Right modified radical mastectomy including level 2 right axillary lymph node dissection was performed [Table/Fig-2]. Histopathology was reported as mixed mucinous carcinoma, having 60% mucinous component and 40% infiltrating ductal component {Scarff Bloom Richardson (SBR) grade 2} with apocrine differentiation [Table/Fig-3,4]. 13 Lymph nodes were identified. All 13 nodes and all the margins were free of tumour. On immunohistochemistry tumour was strongly Estrogen Receptor (ER) and Progesterone Receptor (PR) positive (in 66-100% tumour cells) and moderately positive for Her2neu (>30% tumour cells). The patient received adjuvant hormonal therapy in the form of Tamoxifen and Transtuzumab for six months. Systemic chemotherapy was deferred in this patient due to increased age and poor performance score for its tolerance (as per Radiation Oncologists advice). The patient is on regular follow-up with no evidence of local recurrence or distant metastasis in the past one year.
Preoperative image showing lump in right breast.
Excised specimen after right modified radical mastectomy.
Specimen showing mucinous cells within the ductal component cells H&E stain, 10X.
Specimen showing ductal tumour cells breaching the basement membrane, invasive ductal adenocarcinoma H&E stain, 10X.
Discussion
Male breast cancer is a rare disease accounting for less than 1% of malignancies in men and 0.6% of all breast carcinoma [1]. Male breast carcinoma incidence increases with advancing age, presenting at older age than women. Genetic factors such as BRCA 2 mutation, Klinefelter syndrome and a family history of breast carcinoma are associated with high risk of male breast cancer. Conditions like obesity, reduced testicular function (mumps orchitis, inguinal herniorrhaphy, undescended testis) and radiation exposure which cause hormonal imbalance contributes to epidemiological risk factors for male breast cancer. Gynaecomastia, occupational exposures, prostate cancer, dietary factors, alcohol intake included under suspected epidemiological risk factors. The most common type (90%) of male breast cancer is infiltrating ductal variety [2]. Male breast cancer is diagnosed in advanced stage because of decreased awareness and delayed medical attention sought for breast masses as compared to women [3].
Mucinous carcinoma of male breast is a rare variety of malignancy which accounts for less than 2% of male breast cancers. It can be further be subclassified as mixed or pure forms histopathologically [4]. More than 90% of mucinous cells are seen in pure form of mucinous cancer. They can be further classified as type A (paucicellular) and B (hypercellular) based on their cellular element [5]. Grossly, the tumour is large with rounded outline and is soft in consistency. The cut surface has a characteristic glistening gelatinous appearance. Microscopically, pure form is characterised by tumour composed of malignant cells showing little pleomorphism and low mitotic rate; the cells are set within pools of extracellular mucin surrounded by bands of fibrous connective tissue. Mixed form is inclusive of both mucinous and conventional infiltrating ductal carcinoma component [4]. Mixed mucinous carcinoma has poor prognosis compared to pure mucinous carcinoma. On mammography, pure mucinous carcinoma usually presents as a round and well circumscribed lesion. Presence of non mucinous components is seen as irregular and infiltrating margins on mammogram [6]. On breast ultrasonography, the tumour has well defined margins and it is isoechogenic relative to the fat surrounding the breast tissue [7,8]. The lymph node metastasis incidence in pure mucinous carcinoma is very low than mixed type, accompanied by a favourable prognosis [9]. The presence of extracellular mucin in large quantity acts as a barrier and weaken tumour cell burden in mucinous carcinoma at the invasive margin [1]. Usually pure mucinous tumours are stain positive for ER/PR and negative for HER2neu and mixed forms stain the vice versa. Hence, mixed mucinous tumours have high risk of recurrences and carry a poor prognosis when compared to pure forms.
Mixed mucinous carcinoma in men presents clinically as painless progressive lump as in women. Since the amount of fibrofatty tissue in male breast is far less compared to female breast, a large portion of male breast carcinoma patients have muscle and skin invasion by the tumour at presentation. Though, lymph nodal involvement is less common in patients with pure mucinous carcinoma in men, mixed mucinous tumour has higher incidence of node involvement. Pathologically mixed mucinous tumour in men is characterised by the presence of mucin (50-90%) apart from infiltrating ductal carcinomatous cells. Characteristically these are known to stain less for hormone receptor as compared to pure variety [5].
A mixed mucinous carcinoma should be treated in the same manner as an infiltrating ductal carcinoma. The standard treatment protocol for male breast cancer is surgery (modified radical mastectomy, breast conservative surgery combined with axillary lymph node dissection) followed by chemotherapy, radiotherapy or hormone therapy. The five year disease free survival rate ranged from 81% to 94% (the latter if node negative) and overall five year survival rate of 80% (86% if node negative). Mucinous carcinoma has a risk for late recurrences (i.e., >10 years) with a greater nodal burden [1]. The effect of neuroendocrine differentiation has a positive correlation with favourable histologic/prognostic parameters including low nuclear grade, negative lymph node status and positive PR and negative HER2neu status [1,5].
A mixed mucinous carcinoma occurring in a male breast is a rare entity, not described in most literatures from the past. In the present case, proper preoperative diagnosis was obtained by following principles of breast carcinoma management guidelines. Modified radical mastectomy was performed as patient was not keen on undergoing sentinel node biopsy. Following surgery, adjuvant hormonal therapy was started, considering his age, performance status and the low risk he possessed (absent nodal spread) for distant metastasis. Best results would be obtained because of proper preoperative diagnosis and anticipation of such clinical entities.
Conclusion
Mixed mucinous and infiltrating ductal carcinoma is extremely rare. It usually has unfavourable prognosis. Early detection and aggressive treatment offers better survival benefit to the patient. Clinical stage and axillary lymph node status are of major prognostic value. Increased awareness would result in its early detection and treatment.
[1]. Paramo JC, Wilson C, Velarde D, Giraldo J, Poppiti RJ, Mesko TW, Pure mucinous carcinoma of the breast: is axillary staging necessary?Annals of Surgical Oncology 2002 9(2):161-64.10.1007/BF0255736811888873 [Google Scholar] [CrossRef] [PubMed]
[2]. Ottini L, Palli D, Rizzo S, Federico M, Bazan V, Russo A, Male breast cancerCrit Rev Oncol Hematol 2010 73(2):141-55.10.1016/j.critrevonc.2009.04.00319427229 [Google Scholar] [CrossRef] [PubMed]
[3]. Fentiman IS, Fourquet A, Hortobagyi GN, Male breast cancerLancet 2006 367(9510):595-04.10.1016/S0140-6736(06)68226-3 [Google Scholar] [CrossRef]
[4]. Bussolati G, Sapino A, Mucinous carcinoma and carcinomas with signet-ring cell differentiation. In: Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ, editorsWorld Health Organization Classification of Tumours of the Breast 2012 Lyon, FranceIARC Press:60-61. [Google Scholar]
[5]. Ishida M, Umeda T, Kawai Y, Mori T, Kubota Y, Abe H, Mucinous carcinoma occurring in the male breastOncology Letters 2014 7(2):378-80.10.3892/ol.2013.173024396451 [Google Scholar] [CrossRef] [PubMed]
[6]. Wilson TE, Helvie MA, Oberman HA, Joynt LK, Pure and mixed mucinous carcinoma of the breast: pathologic basis for differences in mammographic appearanceAm J Roentgenol 1995 165(2):285-89.10.2214/ajr.165.2.76185417618541 [Google Scholar] [CrossRef] [PubMed]
[7]. Aggarwal R, Rajni Khanna G, Beg S, Mucinous carcinoma in a male breastJ Cytol 2011 28(2):84-86.10.4103/0970-9371.8075121713154 [Google Scholar] [CrossRef] [PubMed]
[8]. Lam WW, Chu WC, Tse GM, Ruggieri AM, Scola FH, Schepps B, Sonographic appearance of mucinous carcinoma of the breastAmerican Journal of Roentgenology 2004 182(4):1069-74.10.2214/ajr.182.4.182106915039190 [Google Scholar] [CrossRef] [PubMed]
[9]. Kamitani K, Ono M, Toyoshima S, Mitsuyama S, Anan K, Ikeda Y, Isoechoic axillary lymph node metastases of mucinous carcinoma of the breast: a case reportBreast Cancer 2006 13(4):382-85.10.2325/jbcs.13.38217146168 [Google Scholar] [CrossRef] [PubMed]