Compound fractures are fractures which communicate with the external environment through a wound, exposing the bone [1]. Bacterial contamination is reported to occur in 60-70% of the cases which may be responsible for the infectious complications in these patients. The exposed fracture site, presence of devascularised tissue, severity of the external wound, presence of comorbid conditions in the patient, immune status of the patient, delay in arrival to the hospital and delay in treatment are a few reasons that determine the occurrence of infection. Loss of skin integrity and exposure of the subcutaneous tissue provides a warm, conducive environment for the colonization and growth of microorganisms. The presence of foreign body, dead and devitalized tissue in a traumatic fracture wound provide an ideal environment for microbial proliferation and establishment of infection, unless intervened and treated with prophylactic antibiotics and surgical debridement. Once infection is established, wound healing is delayed, treatment cost rises, and the wound management practices become more difficult [2]. Goals of open fracture management include the prevention of infection, achievement of bone union, and the restoration of function.
This study has aimed to predict the appropriate time of culture, its frequency and the type of specimen to be processed for determining the microbial flora of the fracture wound which will help in instituting rational antibiotic treatment for the patient. Diagnostic Microbiology, thus plays a very important role in the effective control of infection and early fracture healing.
To compare the predebridement and debridement cultures in causing postoperative infections in patients with compound fractures of long bones.
Materials and Methods
This was a prospective study conducted for a period of six months from January to June 2016 with a sample size of 100, at the Institute of Microbiology, Madras Medical College, Chennai, Tamil Nadu, India. Institutional ethics committee approval was obtained. Informed consent was obtained from all the patients. Sequential swabs and tissue specimen were collected to isolate and identify the bacteria causing open fractures in long bones. Pre debridement wound swabs were collected within six hours of arrival of the patient to the hospital after thorough wash of the wound. At the time of debridement, in the operating room, a piece of tissue (muscle/skin) was collected in a sterile container with normal saline before antiseptic wash. The patients were followed up for six weeks and in the event of suspicion of an infection, as evidenced by redness, warmth or purulent discharge from the wound, swabs were collected from the wound site. All the samples were plated onto Nutrient agar, 5% Sheep Blood agar and MacConkey agar and incubated for 24 hours at 37°C. Identification of the bacteria was done using standard biochemical tests and antimicrobial sensitivity pattern was determined using Kirby Bauer’s disc diffusion method. Stains, reagents, media and antibiotic discs were procured from Himedia Laboratories, India. The results were analysed using Chi-square tests.
Results
This study was conducted on 100 patients with open fractures of long bones and the results were analysed statistically using SPSS version 17.0.
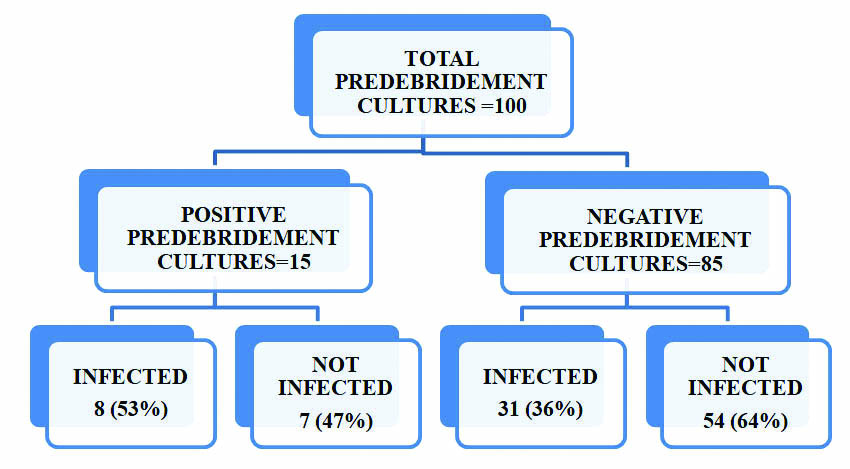
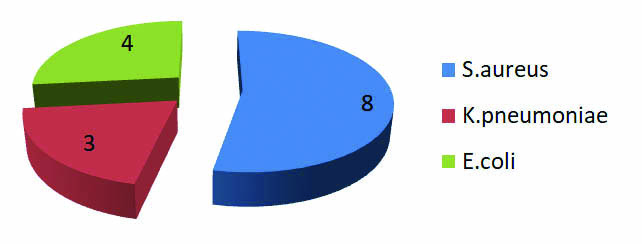
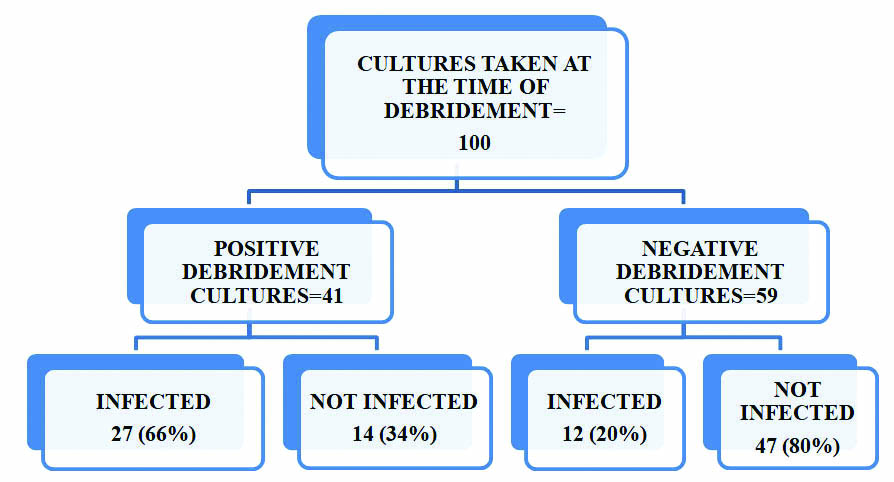
In this study 24% patients were in the age group of 21-30 years of age [Table/Fig-1]. Majority (95%) of the patients were males and 5% were females [Table/Fig-2]. Pre-debridement swabs were collected for all 100 patients on admission, out of which 15% were culture positive. Fifty three percent of pre-debridement culture positive patients developed infection as evidenced by clinical signs and positive culture reports on postoperative follow up [Table/Fig-3].Staphylococcus aureus was isolated in the maximum number (53%) of predebridement cultures, taken immediately on arrival of the patient to the hospital [Table/Fig-4]. A forty one out of 100 cultures taken at the time of debridement showed significant growth, but 27 (66%) patients who were culture positive developed infection on postoperative follow up [Table/Fig-5]. Staphylococcus aureus was the most common organism isolated in 30% of cultures, followed by Pseudomonas aeruginosa in 22% of the cultures [Table/Fig-6]. Thirty nine percent of the patients reported significant growth in the postoperative follow up period [Table/Fig-7].
Age wise distribution (n=100).
| Age in years | Male | Female | Total | Percentage% |
|---|
| 18-20 | 10 | - | 10 | 10% |
| 21-30 | 24 | - | 24 | 24% |
| 31-40 | 19 | 2 | 21 | 21% |
| 41-50 | 21 | 1 | 22 | 22% |
| 51-60 | 19 | 2 | 21 | 21% |
| >60 | 2 | - | 2 | 2% |
Distribution of open fractures in the study population (n=100).
| Limb Affected | Number | Percentage% |
|---|
| Male | Female |
|---|
| Upper limb fractures | 8 | 1 | 9% |
| Lower Limb fractures | 87 | 4 | 91% |
Comparison of predebridement positive cultures and infection in the postoperative period.
Pattern of bacterial isolates in pre debridement cultures.
Comparison of positive debridement cultures and infection in the postoperative period.
Pattern of bacterial isolates in debridement cultures (n=41).
| Organism | Number of Isolates | Percentage% |
|---|
| Staphylococcus aureus | 14 | 30 |
| Klebsiella pneumoniae | 5 | 11 |
| Klebsiella oxytoca | 4 | 9 |
| Escherichia coli | 7 | 15 |
| Proteus mirabilis | 1 | 2 |
| Proteus vulgaris | 1 | 2 |
| Pseudomonas aeruginosa | 10 | 22 |
| Enterobacter aerogenes | 1 | 2 |
| Acinetobacter baumannii | 3 | 7 |
Significant growth reported in 41% of the samples.
Pattern of bacterial isolates in patients with clinical signs of infection in the postoperative period (n=39).
| Organism | Number of Isolates | Percentage% |
|---|
| Staphylococcus aureus | 9 | 22.5 |
| Klebsiella pneumoniae | 6 | 15 |
| Klebsiella oxytoca | 4 | 10 |
| Escherichia coli | 7 | 17.5 |
| Proteus mirabilis | 2 | 5 |
| Pseudomonas aeruginosa | 11 | 27.5 |
| Acinetobacter baumannii | 1 | 2.5 |
Discussion
Hundred patients who sustained open fractures of long bones satisfying the inclusion criteria were included in the study. All the patients had sustained injury due to road traffic accidents.
In this study 24% patients were in the age group of 21-30 years of age, probably due to increased involvement in outdoor activities and road traffic accidents, but the distribution was almost equal from the age of 21 years onwards [Table/Fig-1].
Majority (95%) of the patients were males and 5% were females. This correlated well with the studies by Gupta et al., and Agarwal et al., where the incidence was higher in males than females [1,3]. This may be due to the reason that males are more often involved in driving activities and hence vehicular accidents. Trauma to the lower limb was the commonest (91%), while 9% of patients sustained upper limb fractures [Table/Fig-2]. Sixty one percent of patients reported with fractures of both bones (tibia and fibula) of the leg. This was similar to the observations made by Cole and Bhandari et al., that lower extremity fractures are more common especially [4], open fractures of the tibial shaft which are more prone to get infected due to the high rate of contamination and communition because of its superficial location and subcutaneous characteristics of its anteromedial aspect [5].
In this study, 15% of the cultures were positive in the pre debridement period. Lee et al., in 1997 conducted a retrospective study on 245 open fractures to determine the role of bacterial wound cultures in causing deep infections and observed that, 53% of the pre debridement cultures were positive, which is in contrast to this study where only 15% positive pre debridement cultures were reported [6].
Here, Staphylococcus aureus was isolated in 53% of pre debridement cultures which was almost equal to the rate of isolation of Gram negative bacteria (47%). This may be attributed to the fact that skin commensals which colonize a wound site can subsequently cause infection [Table/Fig-7].
Among the debridement cultures 41% showed significant growth.Staphylococcus aureus was isolated in 30% of the cultures and Pseudomonas aeruginosa in 22%. Members of Enterobacteriaceae were isolated in 46% of debridement cultures.
Postoperative follow up was done for six weeks. Forty six percent of patients showed clinical signs of infection. Thirty nine percent of patients turned out to be culture positive confirming the presence of postoperative infection. Gram negative bacteria contributed to 77.5% and the Gram positive cocci were isolated in 22.5% of the patients. The Gram negative bacteria belonging to Enterobacteriaceae family were the predominant pathogens, accounting for 48% of the cultures. Overall Pseudomonas aeruginosa was the commonest bacteria isolated in 28% of the cultures in the postoperative period. The higher rate of isolation of Pseudomonas aeruginosa in the postoperative period may be due to the production of several virulence factors and also the property to form biofilms adhering to the wound, progressing from colonization to infection [Table/Fig-7]. The incidence of infection proven by culture in the postoperative period in this study was 39%. It is interesting to note that, 53% of predebridement culture positive patients continued to have persistent infection in the postoperative period and 36% with a negative pre-debridement culture also developed infection. On analysing the debridement cultures it was observed that, 66% of the debridement culture positive patients, developed postoperative infection and 59% of them grew the same organism on follow up cultures.
Fifty three percentage of the pre debridement cultures were positive in the postoperative period and 26% of them grew the same organism. So, among the 39% of patients who developed postoperative infection, only 21% had a positive culture on pre debridement and almost 80% were culture negative at the time of pre debridement. This accounts for 21% sensitivity of pre debridement cultures [Table/Fig-8].
Utility of pre debridement cultures in determining postoperative infection (n=100).
| Pre debridement cultures | Postoperative Infection | Total |
|---|
| Present | Absent |
|---|
| Positive | 8 | 7 | 15 |
| Negative | 31 | 54 | 85 |
| Total | 39 | 61 | 100 |
Sensitivity=20.51%, Specificity=88%
Positive Predictive Value=53%, Negative Predictive Value=64%,
Likewise 66% of debridement cultures ultimately led to infection with 59% reporting growth of the same organism. Hence out of the 39% of patients who got infected, 69% turned out to be culture positive at the time of debridement itself and only 31% had negative debridement cultures. Therefore, the sensitivity of debridement cultures is 69% [Table/Fig-9]. Our findings correlated well with the study by Gupta et al., who concluded that debridement cultures are sensitive in predicting the occurrence of a postoperative infection [1]. Our findings were similar to the studies conducted by Lee, Faisham et al., and where pre debridement cultures were of little value in predicting infection [6,7]. Fractures were graded according to the classification by Gustillo and Anderson [8]. The infection rates in the postoperative period were correlated with the fracture grading and it was observed that 40% of Grade IIIB fractures and 26% of Grade I fractures developed infection [Table/Fig-10].
Utility of debridement cultures in determining postoperative infection (n=100).
| Debridement cultures | Postoperative infection | Total |
|---|
| Present | Absent |
|---|
| Positive | 27 | 14 | 41 |
| Negative | 12 | 47 | 59 |
| Total | 39 | 61 | 100 |
Sensitivity=69.23%, Specificity=77%; Positive Predictive Value=66%; Negative Predictive Value=80%
Analysis of cultures based on fracture grading.
| Grade of Fracture | Total | Predebridement Culture Positive | Debridement Culture Positive | Postoperative Infection |
|---|
| Grade I | 15 | 1 | 6 | 4 (26%) |
| Grade II | 33 | 7 | 12 | 12 (36%) |
| Grade IIIa | 9 | 1 | 3 | 6 (67%) |
| Grade IIIb | 43 | 6 | 20 | 17 (40%) |
Antimicrobial susceptibility testing was performed on Mueller Hinton agar by Kirby Bauer’s disc diffusion method. Antimicrobial Susceptibility Pattern of Gram Positive Bacteria is shown in [Table/Fig-11,12,13 and 14]. In this study, 77% of the Staphylococcus aureus isolates were sensitive to Amikacin [Table/Fig-14]. Also, 68% of Staphylococcus aureus showed susceptibility to erythromycin and 61% to ciprofloxacin, but only 16% were susceptible to cotrimoxazole [Table/Fig-14]. Antimicrobial susceptibility pattern of gram negative bacteria-enterobacteriaceae is shown in [Table/Fig-15,16,17 and 18]. Members of the Enterobacteriaceae family showed 100% susceptibility to imipenem, 71% to amikacin and 69% susceptibility to ciprofloxacin [Table/Fig-18]. Antimicrobial susceptibility pattern of gram negative bacteria-non-fermenters is shown in [Table/Fig-19,20 and 21] . Among the Nonfermenters, 100% of Pseudomonas aeruginosa and Acinetobacter baumannii were susceptible to Imipenem and 96% susceptible to Piperacillin-tazobactam and ceftazidime [Table/Fig-21].
Pre debridement culture (gram positive bacteria).
| Organism | AK% | CIP% | PEN% | ERYTHRO% | COTRI% | CEFOX% |
|---|
| Staphylo-coccus aureus (n=8) | (n=6) 75% | (n=4) 50% | (n=7) 88% | (n=6) 75% | (n=2) 25% | (n=7) 88% |
Debridement culture (gram positive bacteria).
| Organism | AK% | CIP% | PEN% | ERYTHRO% | COTRI% | CEFOX% |
|---|
| Staphylo-coccus aureus (n=14) | (n=11) 79% | (n=9) 64% | (n=6) 43% | (n=9) 64% | (n=2) 14% | (n=6) 43% |
Postoperative follow up culture (gram positive bacteria).
| Organism | AK% | CIP% | PEN% | ERYTHRO% | COTRI% | CEFOX% |
|---|
| Staphylo-coccus aureus (n=9) | (n=7) 78% | (n=6) 67% | (n=5) 56% | (n=6) 67% | (n=2) 22% | (n=5) 56% |
Percentage sensitivity of gram positive bacteria to antibiotics.
| AK% | CIP% | PEN% | ERYTHRO% | COTRI% | CEFOX% |
|---|
| 77.4% | 61% | 58% | 68% | 16% | 58% |
PEN: Penicillin; ERYTHRO: Erythromycin; CEFOX: Cefoxitin; AK: Amikacin; CIP: Ciprofloxacin; COTRI: Cotrimoxazole
Pre debridement culture (gram negative bacteria-enterobacteriaceae).
| Organism | CTX% | AK% | CIP% | COTRI% | IPM% |
|---|
| Escherichia coli (n=4) | (n=3) 75% | (n=2) 50% | (n=1) 25% | (n=1) 25% | (n=4) 100% |
| Klebsiella pneumoniae (n=3) | (n=2) 67% | (n=2) 67% | (n=2) 67% | (n=2) 67% | (n=3) 100% |
Debridement culture (gram negative bacteria-enterobacteriaceae).
| Organism | CTX% | AK% | CIP% | COTRI% | IPM% |
|---|
| Escherichia coli (n=7) | (n=3) 43% | (n=5) 71% | (n=5) 71% | (n=4) 57% | (n=7) 100% |
| Klebsiella pneumoniae (n=5) | (n=3) 60% | (n=4) 80% | (n=4) 80% | (n=0) 0% | (n=5) 100% |
| Klebsiella oxytoca (n=4) | (n=1) 25% | (n=3) 75% | (n=3) 75% | (n=1) 25% | (n=4) 100% |
| Proteus mirabilis (n=1) | (n=0) 0% | (n=0) 0% | (n=1) 100% | (n=1) 100% | (n=1) 100% |
| Proteus vulgaris (n=1) | (n=0) 0% | (n=0) 0% | (n=1) 100% | (n=0) 0% | (n=1) 100% |
| Enterobacter aerogenes (n=1) | (n=0) 0% | (n=1) 100% | (n=1) 100% | (n=1) 100% | (n=1) 100% |
Postoperative follow up culture (gram negative bacteria-enterobacteriaceae).
| Organism | CTX% | AK% | CIP% | COTRI% | IPM% |
|---|
| Escherichia coli (n=7) | (n=4) 57% | (n=6) 86% | (n=6) 86% | (n=2) 29% | (n=7) 100% |
| Klebsiella pneumoniae (n=6) | (n=1) 17% | (n=6) 100% | (n=4) 67% | (n=3) 50% | (n=6) 100% |
| Klebsiella oxytoca (n=4) | (n=1) 25% | (n=3) 75% | (n=3) 75% | (n=1) 25% | (n=4) 100% |
| Proteus mirabilis (n=2) | (n=0) 0% | (n=0) 0% | (n=0) 0% | (n=0) 0% | (n=2) 100% |
Percentage sensitivity of gram negative bacteria-enterobacteriaceae to antibiotics.
| CTX% | AK% | CIP% | COTRI% | IPM% |
|---|
| 40% | 71% | 69% | 33% | 100% |
CTX: Cefotaxime; AK: Amikacin; CIP: Ciprofloxacin; COTRI: Cotrimoxazole; IPM: Imipenem
Debridement culture (nonfermentative gram negative bacteria).
| Organism | PT% | CAZ% | AK% | CIP% | COTRI% | IPM% |
|---|
| Pseudomonas aeruginosa (n=10) | (n=10) 100% | (n=10) 100% | (n=7) 70% | (n=3) 30% | NT | (n=10) 100% |
| Acinetobacter baumannii (n=3) | (n=3) 100% | (n=3) 100% | (n=2) 67% | (n=2) 67% | (n=1) 33% | (n=3) 100% |
Postoperative follow up culture (nonfermentative gram negative bacteria).
| Organism | PT% | CAZ% | AK% | CIP% | COTRI% | IPM% |
|---|
| Pseudomonas aeruginosa (n=11) | (n=10) 91% | (n=10) 91% | (n=4) 36% | (n=4) 36% | NT | (n=11) 100% |
| Acinetobacter baumannii (n=1) | (n=1) 100% | (n=1) 100% | (n=1) 100% | (n=1) 100% | (n=0) 0% | (n=1) 100% |
Percentage sensitivity of nonfermentative gram negative bacteria to antibiotics.
| PT% | CAZ% | AK% | CIP% | COTRI% | IPM% |
|---|
| 96% | 96% | 56% | 40% | 25% | 100% |
AK: Amikacin; CIP: Ciprofloxacin; COTRI: Cotrimoxazole; IPM: Imipenem; PT: Piperacillin-Tazobactam; CAZ: Ceftazidime; NT: Not tested
Conclusion
Open fracture wounds are at a high risk of developing infections and other related complications. The management of open fractures is targeted on effective wound debridement, appropriate antimicrobial therapy and early wound closure.
Diagnostic Microbiology plays a crucial role in the control of infection. Cultures taken at the time of debridement are found to be more sensitive in predicting the infection rate compared to the pre debridement cultures. Though several studies have questioned the validity of sequential cultures, this study has proved that debridement cultures have a definite role in predicting postoperative infection.
It is therefore recommended that debridement culture will provide guidance regarding the choice of antimicrobial therapy, which when combined with a thorough wound debridement will enable an early wound closure and lesser complications.
Significant growth reported in 41% of the samples.
Sensitivity=20.51%, Specificity=88%
Positive Predictive Value=53%, Negative Predictive Value=64%,
Sensitivity=69.23%, Specificity=77%; Positive Predictive Value=66%; Negative Predictive Value=80%
PEN: Penicillin; ERYTHRO: Erythromycin; CEFOX: Cefoxitin; AK: Amikacin; CIP: Ciprofloxacin; COTRI: Cotrimoxazole
CTX: Cefotaxime; AK: Amikacin; CIP: Ciprofloxacin; COTRI: Cotrimoxazole; IPM: Imipenem
AK: Amikacin; CIP: Ciprofloxacin; COTRI: Cotrimoxazole; IPM: Imipenem; PT: Piperacillin-Tazobactam; CAZ: Ceftazidime; NT: Not tested