Resin-dentin bonding is a unique form of tissue engineering in which the demineralised collagen acts as a scaffold for resin infiltration to form the hybrid layer [1]. When the depth of dentin demineralisation exceeds the capacity of infiltration of resin monomers, a resin-free demineralised dentin zone is formed at the base of the hybrid layer. The presence of denuded collagen fibrils in this zone is reported to be observed in both total etch and self-etch adhesive systems [1-6]. Hence, though the initial bond strength achieved with contemporary hydrophilic dentin bonding systems is clinically more than adequate, there is a general consensus that these bonds deteriorate over a period of time [7-9].
Hydrolysis of the interfacial components is said to be the main cause for degradation. The infiltrated water in the resin matrix swells and reduces frictional forces between the polymer chains leading to plasticization. This water-driven process decreases the mechanical properties and eludes the breakdown products weakening the bonded interface [10]. Moreover, evidence has demonstrated that degradation can also occur due to the activation of host-derived proteases [11-16].
Dentin matrix contain proteolytic enzymes, the MMPs such as MMP-2,3,8, and -9 which are developmentally secreted as inactive proenzymes. These host-derived proteases constitute a large family of zinc and calcium dependent endopeptidases that participate in many physiological and pathological processes [17]. In addition, non-collagen-bound MMPs are present in dentinal tubules, and presumably in dentinal fluid [18-20].
Apart from MMPs, cysteine cathepsins, papain-like peptidases which exist as endopeptidases and exopeptidases are also present in the dentin matrix. Literature reports the participation of cysteine cathepsins in intra-cellular proteolysis and extra-cellular matrix degradation by the breakdown of type I collagen and proteoglycans [21,22]. Cathepsins B, L and S cleave the non-helical telopeptide extensions of the collagen molecules, while cathepsin K cleaves the collagen molecules along their triple helix region. Unlike MMPs, which cleave collagen at a single site, cathepsin K cleaves collagen molecules of triple helix at multiple sites [23,24]. These proteases are uncovered and get activated only during chemical insult, heat treatment and low pH [25].
Following bonding procedures, MMPs may get activated in three ways; firstly, the acidic components of the adhesives directly trigger their release and activation, secondly, increased MMP-2 synthesis and latent forms of MMPs, namely pro-MMPs in human odontoblasts, thereby increasing its availability in the hybrid layer via the dentinal fluid. Thirdly, the activated cysteine cathepsins in turn may also activate the matrix bound MMPs. The acidic components incorporated into etch and rinse, and self-etch adhesives have been reported to trigger the release and activate these enzymes, thus increasing the collagenolytic and genolytic activities of partially demineralised collagen [7]. Moreover, the acid has been shown to increase MMP-2 synthesis and latent forms of MMPs, namely pro-MMPs in human odontoblasts, thereby increasing its availability in the hybrid layer via the dentinal fluid [21,26]. Also, the activated cysteine cathepsins in turn activate matrix-bound MMPs.
Since these proteases are responsible for the degradation of apatite depleted, resin-sparse collagen fibers compromising the longevity of resin dentin bond, prevention of this degradation becomes critical for the durability of the restoration [1]. This brings about the use of substances that can inactivate these enzymes specifically or non-specifically to functionally stabilise the resin dentin bonds.
Several synthetic and natural cross linking agents have been used to inhibit or reduce biodegradation by endogenous proteases [27,28]. The use of Chlorhexidine digluconate (CHX), a broad-spectrum non-specific proteolytic enzyme inhibitor has been previously reported [29]. According to Turk V et al., Cystatin, a protein inhibitor of cysteine cathepsins, isolated from the chicken egg white has also been shown to inactivate the proteases [30,31]. These inhibitors have been used as a pre-treatment procedure, before the application of bonding agents. But incorporation of these inhibitors in dentin bonding agents and its effect on both MMPs and cysteine cathepsins seems to be of great interest and so far there has been no studies comparatively evaluating the same. Hence, the aim of this in-vitro study was to comparatively evaluate the presence of MMPs and cysteine cathepsins in dentin bonded with adhesives incorporated with non-specific inhibitor chlorhexidine and specific inhibitor cystatin. The null hypotheses proposed that these inhibitors did not inhibit MMP or cysteine cathepsin activity.
Materials and Methods
The experimental study was carried out in the Department of Conservative Dentistry and Endodontics, SRM Dental College, Ramapuram, Chennai and CLRI Chennai. The specimens were subjected to observation for a period of 30 days; since it is a qualitative analysis, no inferential statistics was calculated. Further, sample size estimation was not attempted for the same reason. The ethical clearance certificate was obtained from Institutional Ethical Committee SRM Dental College Ramapuram, Chennai. (File no: SRMU/M&HS/SRMDC/2012/M.D.S-PG Student/304)
Preparation of Experimental Groups
Three solutions were prepared: (i) 0.2% of chlorhexidine (Sigma Aldrich, USA, Lot: C9394) was mixed with total etch adhesive (Adper Single bond 2, 3M ESPE, USA, Lot: 51202) and self-etch adhesive (Adper Easy One, 3M ESPE, USA, Lot: 510939) in the ratio of 9:1; (ii) cystatin (Sigma Aldrich, USA, Lot: C0408) was mixed with total etch and self-etch adhesive in the ratio of 9:1; (iii) 0.2% chlorhexidine and cystatin were mixed with total etch adhesive and self-etch adhesive in the ratio of 8:1:1 respectively.
Specimen Preparation
The diagrammatic representation of the specimen preparation is given in [Table/Fig-1].
The diagrammatic representation of the specimen preparation: a) The roots removed by sectioning at CEJ; b&c) the crowns was sectioned using a diamond disc vertically 2 mm from the mesial marginal ridge to visualise the layer of dentin buccolingually; d) The occlusal surface of the teeth were further cut just below the DEJ in order to expose a flat dentin surface; e-i) 1 mm thick disk of middle/deep coronal dentin measuring 3 mm×3 mm of each tooth was obtained using microtome.
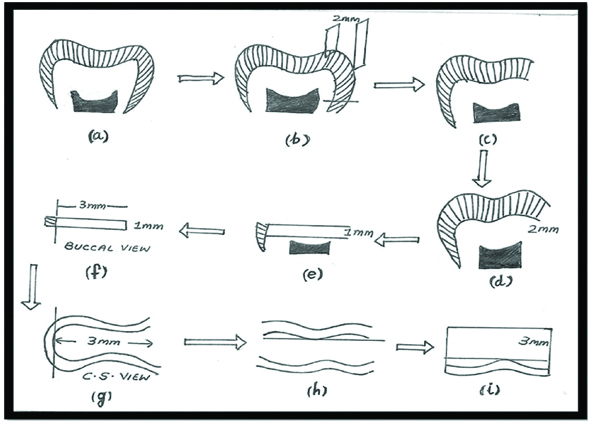
One hundred and thirty-five sound human mandibular third molars scheduled for extraction were extracted after informed consent was obtained, under a protocol approved by the Ethical Committee SRM University, India. The roots of all the teeth were removed by sectioning at CEJ [Table/Fig-1a]. The crown was sectioned using a diamond disc (Indogem Pvt., Ltd., India) under water coolant, vertically 2 mm from the mesial marginal ridge to visualize the layer of dentin bucco-lingually [Table/Fig-1b,c]. The occlusal surfaces of the teeth were further cut just below the Dentino-Enamel Junction (DEJ) in order to expose a flat dentin surface [Table/Fig-1d]. A 1 mm thick disk of middle/deep coronal dentin measuring 3×3 mm of each tooth was obtained using microtome [Table/Fig-1e-i]. The specimens were randomly divided into three groups based on the adhesive systems. The distribution of specimen and the study group are given in [Table/Fig-2].
The distribution of specimen and the study groups.
| Groups | Bonding agents | Sub groups | Zymographic analysis |
|---|
| Group I (n=15) | None | | |
| Group II (n=60) | Total ETCH (TE) | A (n=15) Only bonding agent | 1st, 14th, 30th day (n=5) |
| B (n=15) 0.2% CHX |
| C (n=15) Cystatin |
| D (n=15) 0.2% CHX and Cystatin |
| Group III (n=60) | Self ETCH (SE) | A (n=15) Only bonding agent | 1st, 14th, 30th day (n=5) |
| B (n=15) 0.2% CHX |
| C (n=15) Cystatin |
| D (n=15) 0.2% CHX and Cystatin |
Grouping
Group I (intact dentin): which did not receive any etching or bonding procedure served as control (C; n=15);
Group II: Total etch (TE; n=60), the sectioned dentin surfaces were etched with 37% phosphoric acid gel (Scotch bond universal etchant, 3M ESPE, USA, Lot: 41263) for 15 seconds, rinsed with water and blot dried with dry absorbent paper. Two coats of adhesive were applied and light cured (3M ESPE, USA) for 20 seconds.
Group III: Self-etch (SE; n=60), two coats of self-etch adhesive was applied to dentin surfaces and light cured for 20 seconds.
Both the experimental groups were further subdivided into 4 subgroups (n=15 each) based on the protease inhibitors;
Subgroup A: only bonding agent.
Subgroup B&C: 0.2% chlorhexidine and cystatin were mixed with the bonding agents respectively in the ratio of 9:1.
Subgroup D: Bonding agent were mixed with both 0.2% chlorhexidine and cystatin in the ratio of 8:1:1 respectively.
Following the bonding procedure, all the samples were stored in artificial saliva (Aqwet, Cipla, India, Lot: CP2539) until further analysis. The 135 specimens were pulverised into powder individually. Dentin powder were then demineralised (at 4°C for 24 hours while being stirred) in 0.87 M acetic acid (pH=2.3).
Enzyme Extraction of Demineralised Dentin Specimens and Sample Conditioning
The dentin was pulverised and demineralised into powder form. The powder was then allowed to suspend in extraction buffer containing (50 mM Tris-HCl, pH 6.0, containing 5 mM CaCl2, 100 mM NaCl, 0.1% Triton X-100, 0.1% NONIDET P-40, 0.1 mM ZnCl2, 0.02% NaN3) and EDTA-free protease inhibitor cocktail (Sigma Chemical, St. Louis, MO, USA). The samples were further ultrasonicated and were treated at the output of 40 W (Sonicator Ultrasonic Liquid Processor Model W-385, Heat Systems-Ultrasonic Inc., Farmingdale, NY, USA) for 3 bursts/10 seconds each at 4°C. The supernatants were obtained after the vials were centrifuged for 30 min at 18,000 rpm maintaining the temperature at 4°C. The supernatant that contained protein were allowed to precipitate by adding powdered ammonium sulphate (w/v). This procedure was done to achieve 85% of final concentration with pH 7.0. The precipitate was collected and was redissolved in extraction buffer, in a 10-fold dilution, following centrifugation at 24,000 rpm for 30 minutes at the temperature of 4°C. It was dialysed through a 30-kDa membrane overnight, and was stored at 4°C until analysed [32].
Gelatin Zymography
The dentin proteins specimen that were obtained from enzyme extraction method were subjected to electrophoresis. The electrophoresis was carried out under non-reducing conditions on 7.5% SDS-polyacrylamide gels which is copolymerised with 2 g/L gelatin obtained from porcine skin (Sigma Chemical). The obtained gels from electrophoresis were washed in 2.5% Triton-X 100, agitated and incubated in enzyme incubation buffer (50 mMTris-HCl, pH 7.5, containing 5 mM CaCl2, 100 mMNaCl, 0.01% Triton X-100, 0.1 mMZnCl2, 0.2% Brij non-ionic detergent, and 0.002% NaN3) for minimum of 24 hours at 37°C. Zymographic gels were stained in 0.2% Coomassie Brilliant Blue R-250 and de-stained. Wet gelatin zymograms were scanned at 600 nm by means of a densitometer equipped with an image analyser [32]. Of the fifteen specimens from each subgroup, five samples each were zymographically analysed on 1st, 14th and 30th days.
Results
According to the results obtained in the study, control group showed absence of gelatinolytic activity on all days.
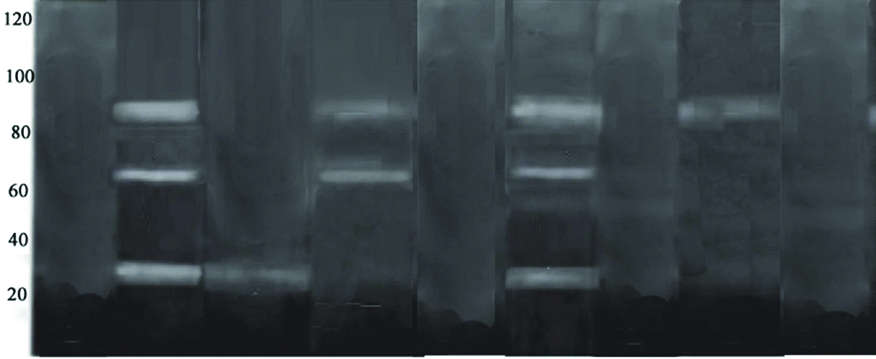
On day 1 [Table/Fig-3] subgroup A of both experimental groups showed presence of both MMPs and cysteine cathepsin activities. In subgroup B (CHX) a faint gelatinolytic activity of cysteine cathepsin at 25 kDa alone was evident only in the group II-TE while it was completely absent in group III-SE group. In subgroup C (cystatin), of both Group II and III only MMP activity at 66 kDa and 86 kDa respectively was evident and cysteine cathepsin activity was completely absent. In subgroup D (chlorhexidine and cystatin) of both Group II and III, gelatinolytic activity was completely absent.
Zymographic analysis on 1st day.
Group I-Absence of gelatinolytic bands indicating no enzymatic activity.
Group II A-Presence of gelatinolytic bands at 66 kDa, 86 kDa, 25 kDa indicative of MMP-2, MMP-9 and cysteine cathepsin activity respectively.
Group II B-Presence of gelatinolytic band only at 25 kDa indicative of cysteine cathepsin activity only.
Group II C-Presence of gelatinolytic band at 66 kDa and 86 kDa indicative of MMP-2 and MMP 9 activity.
Group II D-Absence of gelatinolytic activity indicating no enzymatic activity.
Group III A-Presence of gelatinolytic bands at 66 kDa, 86 kDa and 25 kDa indicative of MMP-2, MMP-9 and cysteine cathepsin activity.
Group III B-Absence of gelatinolytic bands indicating no enzymatic activity.
Group III C-Presence of gelatinolytic bands only at 86 kDa indicative of MMP-9 activity.
Group III D-Absence of gelatinolytic bands indicating no enzymatic activity.
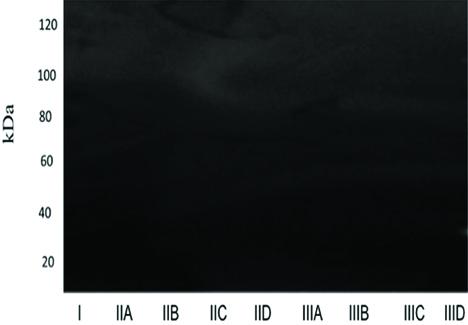
On the 14th and 30th days the absence of gelatinolytic bands in all the experimental groups was evident [Table/Fig-4,5].
Zymographic analysis on 14th day. Gelatinolytic band was not evident in any of the groups, indicating absence of enzymatic activity.
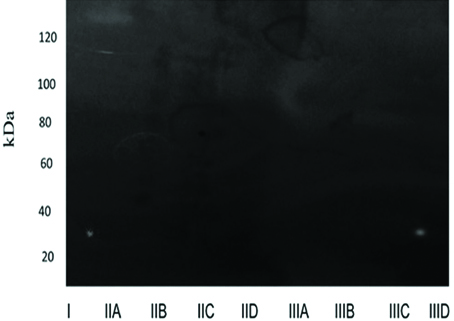
Zymographic analysis on 30th day. Gelatinolytic band was not evident in any of the groups, indicating absence of enzymatic activity.
Statistical Analysis
Descriptive analysis was calculated and expressed in terms of frequency. Since there were no numerical differences in the groups at any time point, there was no scope for calculating inferential statistics.
Discussion
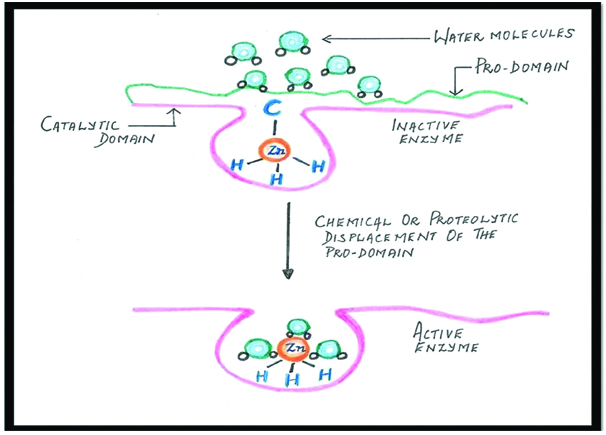
The potential role of host-derived proteases in dentin matrix degradation has been reported in the literature [1,11-16]. MMPs, the endogenous Zn2+-and Ca2+-dependent enzymes are a mammalian family of ECM which are known for their participation in normal dentin formation [1]. After mineralization of dentin matrix, these enzymes remain trapped in the matrix, either in active or proenzyme forms (latent form) [33]. MMPs remain inactive as long as the bond between cysteine residue (Cys73) in the propeptide domain of the MMPs, and the active Zn2+ site in the catalytic domain are stable. When this Cys73–Zn2+ bond is disrupted, the MMPs get activated which is referred to as the “cysteine switch” [Table/Fig-6] [34]. It is being hypothesised that during low pH, it re-exposes and causes conformational changes in the propeptide domain of the enzyme, facilitating activation of cysteine switch, which results in fully activated enzyme [35].
Schematic representation of “Cysteine switch”.
Cysteine cathepsins, another enzyme family have also been identified in the dentin, which has been claimed to be comparable to that described for MMPs. Cysteine cathepsins are members of the C1 family of papain-like enzymes, the largest and the best characterised family of cysteine peptidases [36,37]. The extracellular matrix consisting mostly of collagen and proteoglycans, have been identified as the cathepsin substrates [23,25].
Activation of cysteine cathepsin activity and conversion into its mature form, at neutral pH is regulated by Glycosminoglycans (GAGs) [25,38]. During acid-demineralization cysteine cathepsins activate MMPs by proteolytic inhibition of TIMPs. Moreover, procathepsin B can be activated by active MMPs, and conversely, cathepsin B has been shown to be responsible for activation of MMP-1 in gingival fibroblast cultures. This concludes that both class of proteolytic enzymes, cysteine cathepsins and MMPs, participate synergetically in dentin-pulp complex and plays a role in the degradation of the hybrid layer collagen, created by contemporary etch and rinse and self-etch adhesives [19-21,23,25]. Breschi L et al., stated that these enzymatic activity were predominant at the bottom of the hybrid layer, which correlates well with the layer of uninfiltrated collagen [8].
Hence, treatment strategies based on inhibiting endogenous dentin proteases in preservation of collagen matrix integrity is crucial for creating a stable adhesive restoration. In most of the previous studies, protease inhibitors are applied to the etched dentin surface as a pre-treatment procedure before resin infiltration, but this method may not hold good for self-etch adhesives because of the concurrent demineralization and infiltration [39]. Moreover the introduction of an additional step may increase the chair side time, which may not be favourable. However, no data concerning the comparative effect of adhesive incorporated inhibitors on host proteases are available in the literature [14]. Thus in this study, chlorhexidine and cystatin a non-specific and specific inhibitor respectively were incorporated in total etch and self-etch adhesives to evaluate the presence of MMPs and cysteine cathepsins in dentin.
In the present study, 0.2% CHX was mixed with the adhesives in a ratio of 9:1. The concentration of chlorhexidine was kept relatively low, to minimise the interference with the well-balanced monomer combination of the adhesives [29]. However, the chlorhexidine concentration was still higher than the minimal amount of chlorhexidine needed for complete inhibition (0.0001% and 0.002% for MMP-2 and MMP-9, respectively) [39,40].
Cystatin, an egg white protein is a potent inhibitor with a minimum inhibitory concentration in the nanomolar range [30,36]. In this study, it was mixed with the adhesive in a ratio of 9:1.
A method that does not impede desired specificity between the structurally similar family members of MMPs namely zymography or substrate gel electrophoresis is employed in this study to detect the activity of MMPs and cathepsins [41,42].
Enzymes exist in their pro-form throughout without being evident and can be activated only in a low pH environment. This confirms with the results obtained in this study, in which there was no evidence of enzyme activity in Group-I. In Group II TE-A and Group III SE-A, the enzymatic activity of both MMP and cysteine cathepsin were evident. This can be attributed to the reactivation of gelatinases (MMP-2 and MMP-9), collagenases and latent MMPs in demineralised dentin by low pH of total etch (2.7) and the acidic monomers (pH -1.5-2.7) in SE adhesives, resulting in a 14-to 15-fold increase in collagenolytic activities [8,40].
Comparing groups II TE-B and III SE-B the total etch group, a faint gelatinolytic activity of cysteine cathepsin, with complete inhibition of MMP activity was observed. In total etch adhesives, the initiation of MMP activation starts with the application of phosphoric acid with its pH being 0.8 and when the adhesives with the pH 2.7 is again applied subsequently, it could have further aggravated activation of the enzymes by acid activation mechanism, which might explain the inability of chlorhexidine to completely inhibit the enzymes in this study. In Group III SE-B (self-etch adhesives with chlorhexidine only), there was complete inhibition of MMPs and cathepsins activity. The coordination between the hydroxyl group of HEMA and the bivalent cation (ZN2+) that is present in the catalytic domain of MMPs are said to be the possible main mechanism for inhibitory activity [43,44]. The results of this study confirms with the findings of Gendron R et al., [29].
Chlorhexidine is an amphiphilic molecule that binds to several proteins by a cation-chelating mechanism. It prevents the binding of metal ions, such as zinc or calcium in the MMPs and so could inhibit its catalytic activity [39]. CHX binds electrostatically to demineralised dentin collagen and may slowly diffuse out of the collagen matrix via a competitive desorption mechanism in the presence of other mono and divalent cations derived from body fluids. [35] Moreover, Scaffa PM et al., have also confirmed that electrostatic binding between CHX and cathepsins is possible and easily achievable [45].
In groups II TE-C and III SE-C, only cysteine cathepsins activity was inhibited, with evident MMP activity. Because of cystatin’s specificity, the gelatinolytic activity of other enzymes, including MMPs, may not be inhibited by treatment with cystatin. Turk V et al., and Bobek LA et al., have provided evidence that cystatin is a stable, tight binding competitive inhibitor of cysteine proteinases [30,46]. Cystatin inhibition of cysteine peptidases of the papain family is due to a tripartite wedge-shaped structure which acts as active cleft site for the enzymes.
The results of our study showed that the combined use of both inhibitors resulted in a synergistic effect as there was complete inhibition of both MMP and cysteine cathepsin activity in subgroup D.
Chlorhexidine which is an amphiphilic molecule binds to several proteins by a cation chelating mechanism [47]. Cystatin being a protein could have bound electrostatically to chlorhexidine by chelation bringing about the denaturing of protease activity. This chelation in turn forms a precipitated protein that apparently forms a stronger binding of the inhibitors to the enzymes both specifically and non-specifically.
On the 14th and 30th days, no gelatinolytic bands were evident in any of the groups. This could be because of the storage solution i.e., artificial saliva used in this study to age demineralised dentin disk samples. The artificial saliva does not exactly mimic the bodily oral fluids, as it is devoid of Zn and Ca ions. Due to its absence, this media could have underestimated the degrading activity of MMPs and cysteine cathepsin, which reflects the results of the present study, showing no gelatinolytic bands in any of the groups. These results are in concurrence with the results of the study done by Mutluay A et al., [48].
Limitation
The relative abundances of both active and inactive forms of proteases in situ or in vivo should be evaluated to generate a biologically relevant role of the studied proteases.
Conclusion
Under the limitations of this study, it can be concluded that incorporation of both 0.2% chlorhexidine and cystatin in total etch and self-etch adhesives inhibited the MMPs and cysteine cathepsin activity completely, suggesting the stability of the resin dentin bond over a period of time.