Endoscopic Retrograde Cholangiopancreatography (ERCP) is a complex procedure performed on an outpatient basis and plays a crucial role in diagnosis and treatment of pancreaticobiliary pathologies. Mostly the ERCP are performed with the patient under moderate or conscious sedation, deep sedation and general anaesthesia [1-3]. Use of conscious sedation for ERCP is limited. It is hampered by inadequate sedation/analgesia which may result in movement or coughing, disrupting endoscopist’s view and increasing chances of adverse effects [4]. These shortcomings propagate the use of deep sedation monitored by Bispectral Index (BIS) for good success [5]. American Society for Gastrointestinal Endoscopy has also stated that the use of EEG monitoring may have a role in endoscopic procedure [6,7]. The major challenges involved are preservation of spontaneous respiratory efforts, cardiovascular stability, shared airway and positional variations.
There are various agents available to provide deep sedation. The current drug/combination includes benzodiazepines (most commonly midazolam and diazepam) with an opioid (often fentanyl or remifentanil), ketamine, dexmedetomidine with or without propofol [8,9]. The administration of a single agent during ERCP leads to inadequate sedation and analgesia requiring excessive drug use and increase in undesirable side effects. Therefore, combinations of drugs with different pharmacological effects are preferred.
The propofol and midazolam have quick onset, short duration of action and rapid recovery profile, because of these properties these drugs are preferred by endoscopist. The combination of midazolam and opioid causes prolonged recovery, hypoxemia, hypotension and respiratory depression. Propofol does not have analgesic effects, and high doses are often required during painful procedures which may lead to cardiorespiratory adverse effects [10]. Addition of opioid or dexmedetomidine reduced propofol requirements. Dexmedetomidine is a highly selective α-2 adrenoceptor agonist with sedative, analgesic, and opioid-sparing effects, without causing significant respiratory depression [11].
Hence the present prospective, randomized, double blind study was designed to evaluate and compare the efficacy of propofol-fentanyl, propofol-midazolam and propofol-dexmedetomidine combination on haemodynamic and postoperative recovery in ERCP patients.
Materials and Methods
This prospective, randomized, double blind study was conducted after approval from the Institutional Ethics Committee and written informed consent from the patients. The study was conducted at Apollo Hospitals, Bilaspur (CG) India, from June 2015 to March 2016. The study was registered at Clinical Trials.gov www.ctri.nic.in (ref: CTRI/2015/06/005842).
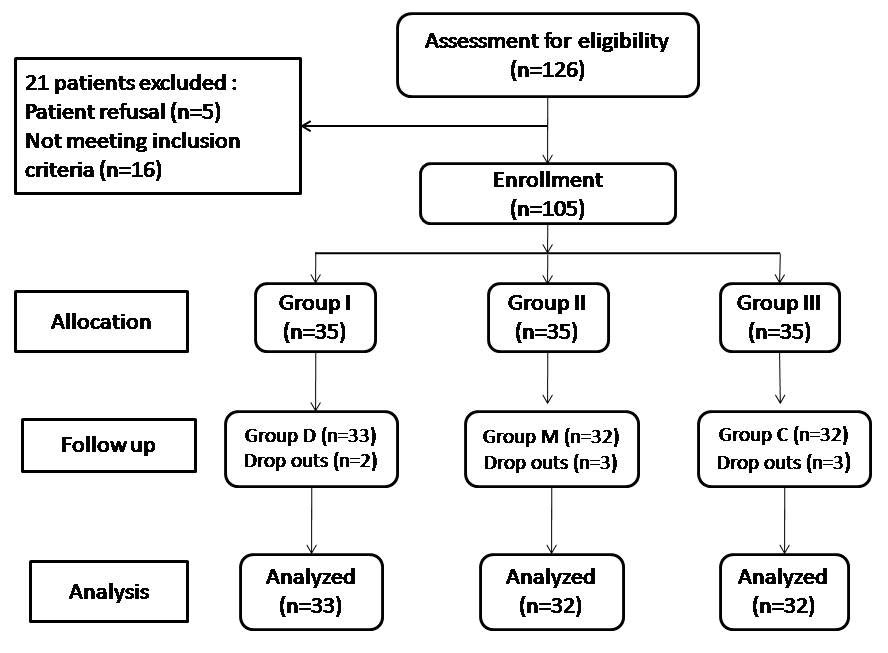
A total of 126 patients, either sex, 20-65 years of age, ASA (American Society of Anaesthesiologists) physical status I and II scheduled for ERCP were included in this study. Patients with comorbid conditions such as diabetes mellitus, hypertension or hepatic or renal insufficiency, hematological disorder or cardiovascular dysfunction, any degree of heart block, chronic use of opioids, sedatives, and current treatment with a β-blocker were excluded from the study. A total of 21 patients were excluded from the study because of patient refusal and not meeting inclusion criteria [Table/Fig-1]. The 105 patients were randomly allocated to three groups of 35 each with the help of a computer generated table of random numbers.
Group I : Fentanyl (Verfen, Verve health care Ltd.) 1 mcg/kg over 10 min, followed by propofol loading dose 1-2 mg/kg before the procedure and maintenance infusion at a rate of 1-5 mg/kg/h throughout the procedure.
Group II : Midazolam (Benzosed, Troikaa pharmaceuticals) 0.04 mg/kg over 10 min, followed by propofol loading dose 1-2 mg/kg before the procedure and maintenance infusion at a rate of 1-5 mg/kg/h throughout the procedure.
Group III : Dexmedetomidine (Dexem, Themis medicare) 1 mcg/kg over 10 minute, followed by propofol loading dose 1-2 mg/kg before the procedure and maintenance infusion at a rate of 1-5 mg/kg/h throughout the procedure.
All the study drugs were prepared in 20 ml normal saline and infused before induction of anaesthesia. These drugs were prepared by an independent anaesthesiologist (SK) not involved in the study. Another anaesthesiologist blinded to the study drugs monitored the patients. The final analysis was done by the “Principal Investigator” only.
In the procedure room, preloading was done with 3 ml/kg of normal saline. Routine monitoring of electrocardiography, pulse oximetry, respiratory rate and blood pressure were established before starting study drug (Philips IntelliVue MP 40 Monitor, Germany). In addition, the electroencephalographic BIS value was obtained using a single channel sensor (BIS QuatroTM, Coviden, Mansfield, MA, USA) in a frontal temporal montage.
After infusion of study drugs, glycopyrrolate 0.2 mg i.v. given to all patients and patients were put in semi-prone position during ERCP. All the patients received oxygen (2-4 L/min) by a nasal prong throughout the procedure. Anaesthesia was induced with propofol in a dose of 1-2 mg/kg, targeting BIS below 60. Once the target BIS was attained, the maintenance infusion of propofol was started. An experienced Gastroenterologist then commenced the procedure. During the procedure, anaesthesia was maintained with propofol infusion to achieve a target BIS between 50 and 70. During the procedure if the patient required more than three episodes of personal restraint by the assistant or if either patient or endoscopist was uncomfortable, or BIS >70, an additional 10-20 mg of propofol bolus was administered. Procedure duration was defined as the time between scope insertion and scope withdrawal. At the completion of the procedure, propofol infusion was stopped. All procedures were conducted by the same endoscopist. The Modified Aldrete Score (MAS; 0-10) was used to assess recovery [12]. Patients were discharged from the recovery room after attaining an MAS of 9–10. Time taken to achieve this score was recorded. At the end of the procedure overall satisfaction of the endoscopist was recorded using a Visual Analog Scale (VAS, 0-100 mm; 0 = no satisfaction and 100 = maximum satisfaction). At discharge, the patient’s satisfaction was also noted using similar VAS.
Perioperative complications were recorded and managed accordingly. Oxygen desaturation or apnea (SpO2<92% or cessation of respiration for 15s or more) were managed by supporting the airway and/or assisting ventilation. Hypotension (decrease in MAP>30% of baseline) was managed by fluid bolus and/or vasopressors. Bradycardia (HR< 50 beats/min) was managed with atropine 0.6 mg i.v. whereas, a HR and MAP more than 30% from the baseline level was considered as tachycardia and hypertension. Other complications such as coughing, gagging, hiccough, nausea and vomiting were also recorded.
Heart Rate (HR), Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), Mean Arterial Pressure (MAP) and Oxygen Saturation (SpO2) were recorded at preoperative, after study drug administration, after induction, during procedure at 1, 3, 5, 10, 15, 20, and 25 minute and after 5 minute of end of the procedure.
After the initial pilot observations, it was decided that a 10% of difference in the heart rate should be the minimum detectable difference of means in all 3 groups. The Standard Deviation (SD) of residual was 14% of average difference of all 3 groups. The alpha value was 0.05 and the power of the study was 0.80. Thus, the calculated sample size was 29 patients per group. Taking into account possible dropout rate of 15% from three groups, we decided to enroll a total of 105 patients.
Statistical analysis was performed using the Graph pad prism 6.0 statistical software. Patient characteristic data were analysed with one-way analysis of variance (ANOVA) for continuous variables and Chi-square test for categorical variables. Intergroup comparison of haemodynamic parameters were done with one-way analysis of variance (ANOVA), followed by an unpaired t-test. Repeated measure analysis of variance (ANOVA) with the post-hoc Tukey test was used to compare means for haemodynamic variables in intragroup comparison to preoperative parameters. Satisfaction score was analysed by the Kruskal-Wallis test. A p-value of <0.05 was considered statistically significant.
Results
A total of 105 patients were included in the study after randomization and 97 patients (92.4%) completed the study [Table/Fig-1]. Eight patients were not included in this study on account of gastric outlet obstruction (one patient in group I and group II each), procedure time less than 15 minute (one patient in group I and group III each), history of hypotension (two patient in group III) which require vasopressors and two patient in group II developed tachycardia and hypertensive response which require administration of esmolol. Their data has been included in the comparison of demographic profile; however, they were not subjected to further statistical analysis.
There was no significant difference amongst the groups with regard to demographic variables (p>0.05) [Table/Fig-2]. The requirement of propofol dose was significantly lower in the group III compared to group II (p<0.05).
| Variables | Group I | Group II | Group III | p-value |
|---|
| Mean age (yr) | 49.11±10.80 | 44.71±12.99 | 46.54±11.42 | 0.295* |
| Weight (kg) | 57.88±8.88 | 55.91±9.05 | 57.50±9.17 | 0.650* |
| Male/Female | 19/16 | 17/18 | 15/20 | 0.915** |
| Duration of procedure (min) | 30.51±5.98 | 30.84±6.91 | 29.81±6.46 | 0.808* |
| Propofol dose (mg) | 276.67±76.06 | 306.88±74.86 | 252.66±69.84 | <0.05* |
Data are presented as either mean values±SD or by absolute numbers.
* Analysed with the ANOVA test; ** Analysed with the Chi-square test.
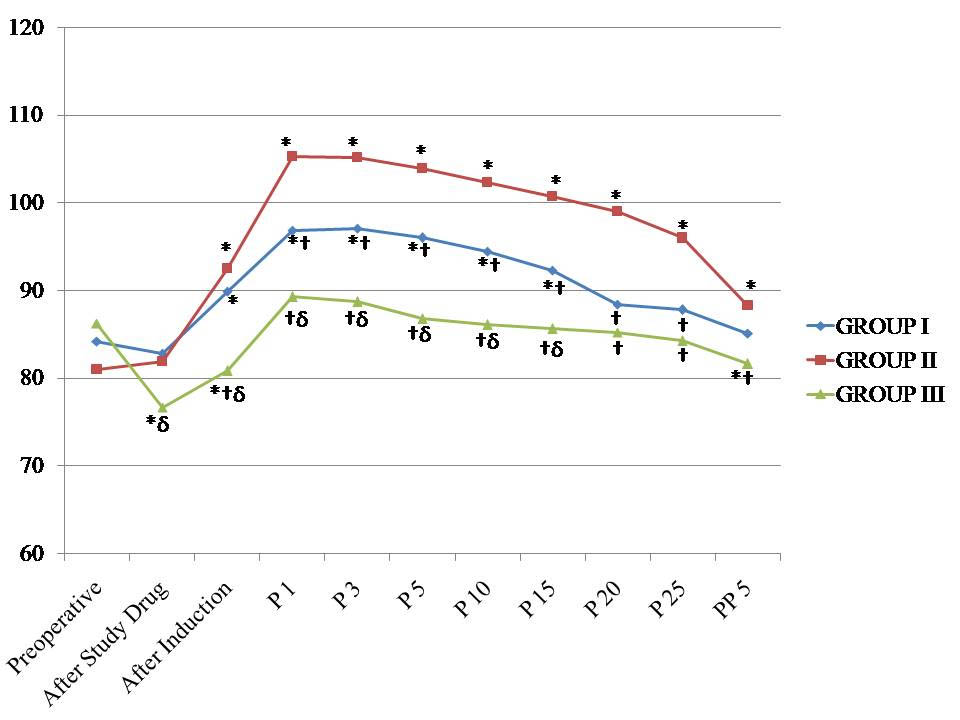
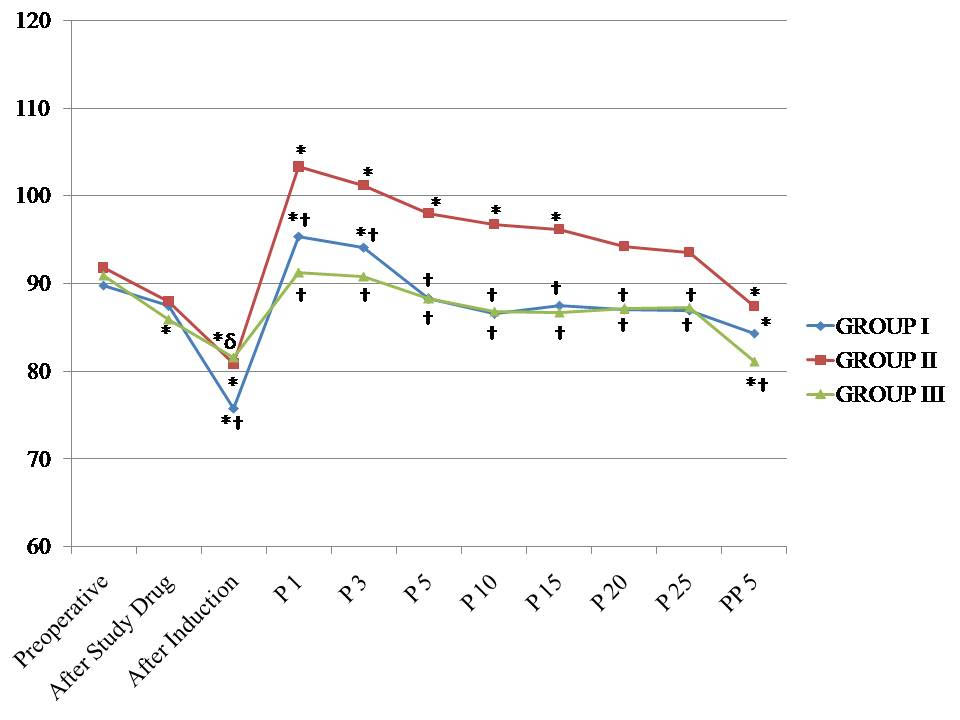
There was no significant difference in preoperative haemodynamic parameters between the groups. After administration of the study drugs, there was a significant decrease in heart rate in group III (p<0.05). After induction, there was no significant difference in HR between groups I and II (p=0.435). There was a significant increase in the heart rate in group II during the whole endoscopic procedure and in the group I, up to 15 minute only (p<0.05), in comparison to preoperative values. In group III, there was no significant increase in HR values during the procedure. There was a significant difference in HR values amongst the groups, except between group I and group III, during 20 minute (p=0.104) and 25 minute (p=0.086) intervals [Table/Fig-3].
Changes in the heart rate observed in the three groups during the study period. *p < 0.05 within group (vs. preoperative value), †p < 0.05 compared with group II, δp < 0.05 group I versus group III. P1 – One minute, P3 – Three minute, P5 – Five minute, P10 – Ten minute, P15 – Fifteen minute, P20 – Twenty minute, P25 – Twenty five minute during procedure and PP5 – Five minute after procedure
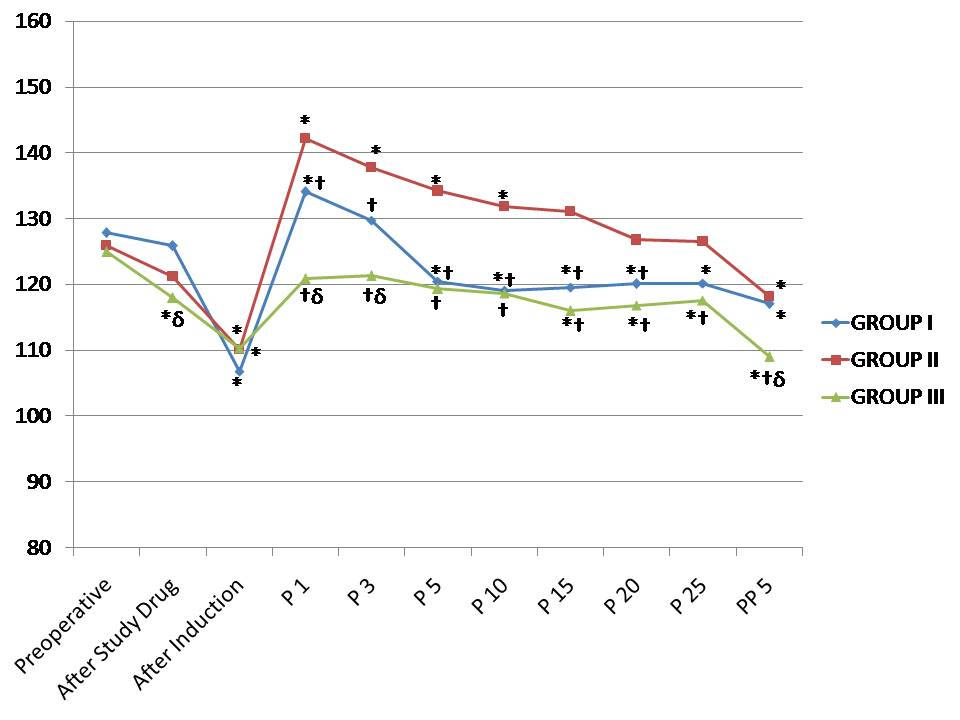
SBP and DBP values were statistically significantly lower in the group III after study drug administration and after induction in comparison to preoperative values (p<0.05). There was no significant increase in SBP and DBP values in group III in comparison to preoperative values during the procedure, while there was a significant increase in group I at 1 minute and 3 minute only and in group II up to 15 minute intervals. There was no significant difference in SBP, between group I and group III during the procedure from 5 minute onwards while DBP values was not significant at any time intervals during the procedure [Table/Fig-4,5].
Changes in the systolic blood pressure observed in the three groups during the study period. *p < 0.05 within group (vs. preoperative value), †p<0.05 compared with group II, δp < 0.05 group I versus group III. P1 – One minute, P3 – Three minute, P5 – Five minute, P10 – Ten minute, P15 – Fifteen minute, P20 – Twenty minute, P25 – Twenty five minute during procedure and PP5 – Five minute after procedure.
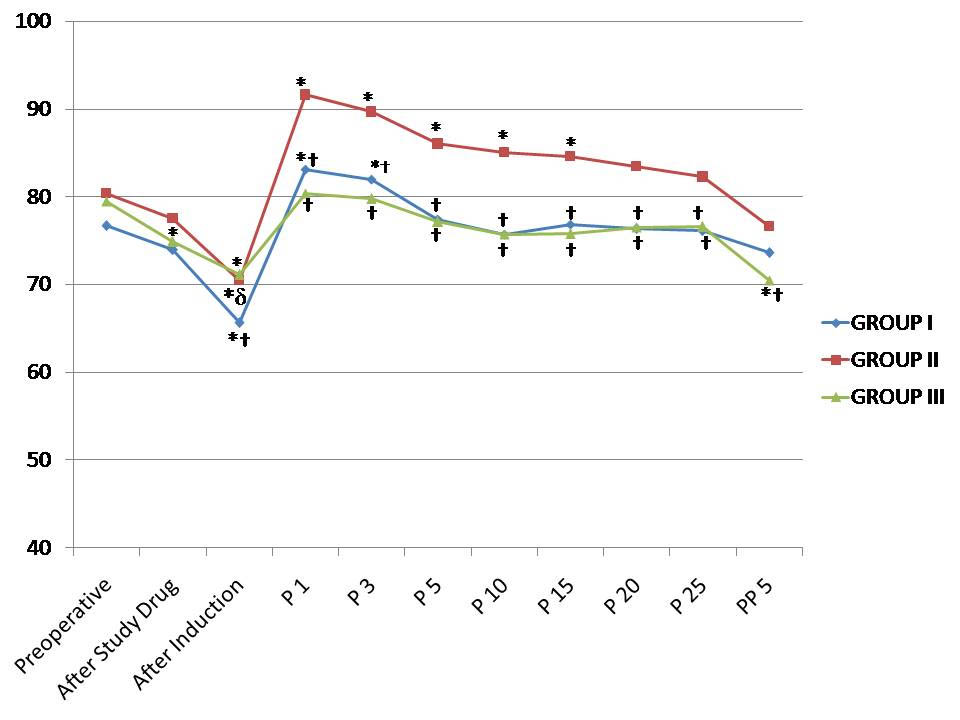
Changes in the diastolic blood pressure observed in the three groups during the study period. *p < 0.05 within group (vs. preoperative value), †p < 0.05 compared with group II, δp < 0.05 group I versus group III. P1 – One minute, P3 – Three minute, P5 – Five minute, P10 – Ten minute, P15 – Fifteen minute, P20 – Twenty minute, P25 – Twenty five minute during procedure and PP5 – Five minute after procedure.
MAP values were statistically not significant compared to preoperative values at any time intervals of procedure in group III, while there was a significant increase in group I at 1 minute and 3 minute only. In group II, there was a significant increase in MAP up to 15 minute intervals of the procedure. There was no significant difference in MAP, between group I and group III during the procedure (p>0.05) [Table/Fig-6].
Changes in the mean arterial blood pressure observed in the three groups during the study period. *p < 0.05 within group (vs. preoperative value), †p < 0.05 compared with group II, δp < 0.05 group I versus group III. P1 – One minute, P3 – Three minute, P5 – Five minute, P10 – Ten minute, P15 – Fifteen minute, P20 – Twenty minute, P25 – Twenty five minute during procedure and PP5 – Five minute after procedure.
Time to achieve MAS to 9-10 was significantly not different among the groups (p=0.053). Endoscopist’s satisfaction was significantly more in group I and group III compared to group II (p<0.05), but patients’s satisfaction was similar among the groups (p>0.05) [Table/Fig-7]. There was no significant difference in SpO2, between the groups during the procedure (p>0.05). The occurrence of coughing, gagging, nausea, hiccough and vomiting were more in group II. Hypotension was observed in two patient (5.7%) in group III, which responded to administration of 2 doses of mephentermine 0.5 mg i.v. Two patient (5.7%) in the group II developed hypertensive response during procedure which was managed with esmolol bolus 0.5-1.0 mg/kg [Table/Fig-8].
Modified aldrete scores (min) and satisfaction score.
| Time Interva | Group I | Group II | Group III | p-value |
|---|
| Time to achieve Aldrete 9-10 | 7.49±2.48 | 8.01±2.54 | 9.06±2.85 | 0.053* |
| Endoscopist satisfaction | 90.30±9.18 | 83.12±11.48 | 93.12±8.21 | <0.05** |
| Patient satisfaction | 91.82±7.69 | 88.44±8.84 | 92.81±9.24 | 0.074** |
Values expressed as mean VAS±standard deviation (SD).
* Analysed with the ANOVA test; **Analysed with the Kruskal-Wallis test.
Complications according to groups.
| Complications | Group I | Group II | Group III |
|---|
| Coughing | 3 (8.57) | 6 (17.14) | 2 (5.71) |
| Gagging | 5 (14.28) | 8 (22.86) | 4 (11.43) |
| Hiccough | 2 (5.71) | 5 (14.28) | 1 (2.86) |
| Nausea/vomiting | 4 (11.43) | 2 (5.71) | 1 (2.86) |
| Hypotension | 0 | 0 | 2 (5.71) |
| Hypertension | 0 | 2 (5.71) | 0 |
Data presented in number (%) of patients.
Discussion
Anaesthesia for ERCP is challenging because of the need for adequate sedation/analgesia and patient safety without any adverse effects. Our study demonstrated that the use of propofol–dexmedetomidine and propofol–fentanyl under BIS guidance provided adequate operative conditions without significant adverse effects. Klopman MA et al., showed that the use of BIS monitoring significantly reduced anaesthetic consumption [13]. Our data also suggest that BIS monitoring led to a reduction in the mean propofol dose when the BIS value was used as the primary target for sedation in ERCP procedures.
Propofol is a preferred drug for sedation during ERCP. Administration of propofol as a sole agent during ERCP leads to inadequate sedation and analgesia and thus to excessive drug use and increase in undesirable side effects. Hence, a combination of propofol and adjuvant (benzodiazepine, opioids, ketamine, dexmedetomidine or cocktail regimens) has been shown to reduce the dose requirement of propofol while maintaining comparable efficacy and analgesia [14]. In our study we used adjuvants, midazolam, fentanyl and dexmedetomidine because of favourable recovery profile characters as well as anaesthetic sparing effects. Muller S et al., noted that as a single agent, dexmedetomidine or midazolam was found less effective in producing conscious sedation during ERCP [15].
Pain during ERCP has been reported to provoke neurohumoral reflexes, cardiovascular aterations and even cardiac arrest [16,17]. In the present study, both the study drugs (dexmedetomidine and fentanyl) in combination with propofol have better haemodynamic stability with minimum fluctuations during whole ERCP. Pain control is mediated by μ recptors with fentanyl while effect on locus cereleus is responsible for action of dexmedetomidine.
Dexmedetomidine usage is associated with a greater decrease in HR, in part because of its sympatholytic effects, but also because of a vagal mimetic effect. The most significant cardiovascular effect of propofol during the induction of anaesthesia is a drop in the blood pressure, seen in all groups of patients. In their study, Srivastava VK et al., administered induction dose of propofol guided by BIS after same loading dose of dexmedetomidine and determined significant decreases in blood pressure levels compared to preoperative value [18]. From start of endoscopic procedure to the end of procedure, MAP remained at a non significant change compared to preoperative value in dexmedetomidine and fentanyl groups than in midazolam group. This probably indicates the analgesic properties of dexmedetomidine and fentanyl. Dexmedetomidine is also known to decrease sympathetic outflow and circulating catecholamine levels and would therefore be expected to attenuate the haemodynamic response to endoscopy [18,19]. In the midazolam group there was a haemodynamic derangement because this group has no analgesic properties.
The most significant cardiovascular effect of dexmedetomidine is hypotension and bradycardia. In our study, we observed hypotension in 5.7% patient. The hypotension and bradycardia that occurred in the dexmedetomidine group were predictable from the known properties of α2 agonists, and have been confirmed from previous studies [18,19]. In midazolam group, we observed tachycardia and hypertension (5.7%) during endoscopy because of no analgesic adjuvant in this group.
In the present study dexmedetomidine group achieved a 20% decrease in propofol requirement compared to midazolam group. Similar effects were observed by other authors also [18,20]. This reduction in dexmedetomidine group is due to the hypnotic, sedative, analgesic and anaesthetic sparing effect of dexmedetomidine.
In our study, there were no statistically significant differences between the groups in terms of the satisfaction of the patients because we guided sedation level by BIS. However, the endoscopist’s satisfaction was higher in dexmedetomidine group than in fentanyl and midazolam group, because dexmedetomidine group patients were more haemodynamically stable, had less coughing, gagging, movement and less bleeding during papillotomy. In our study, MAS was used and no significant differences were determined between the groups. In the study, Ramkiran S et al., administered propofol with combination of dexmedetomidine, ketamine and saline (control) and noticed that the time taken to achieve MAS>9 was significantly longer in the dexmedetomidine group when compared with the control and ketamine groups [9]. This difference is due to the fact that they used dexmedetomidine bolus (1 mcg/kg), followed by dexmedetomidine infusion along with variable propofol boluses.
Most studies [14,20] have noticed the recovery profile, satisfaction score, sedation score and procedure related complications but there are few studies [8,9] that tell us about haemodynamic derangements during ERCP with different drug uses. The advantage of present study is that there is reduction in dose of propofol, lower rate of haemodynamic derangements and desaturation and no patient require bag-mask ventilation or endotracheal intubaion. We hypothesized that by giving continuous infusion of propofol guided by BIS, the constant level of propofol would not exceed the therapeutic window and this in turn might reduce the adverse effects. Jang SY et al., confirmed that if we achieved the desired level of sedation using a minimal dose of propofol with BIS monitoring, then the risk of respiratory depression is reduced [6].
Raymondos K et al., reported premature termination rate of procedure around 14% either due to inadequate sedation or airway related complications [21]. In a retrospective analysis by Goudra B et al., the incidence of cardiac arrest and death is about 10 times higher in patients receiving propofol-based sedation compared with those receiving midazolam–fentanyl sedation [22]. Dexmedetomidine group was most cost effective, because it maximally reduced propofol requirements.
Limitation
Our study did not use advanced monitoring equipment, such as capnography. Capnography is more sensitive than pulse oximetry or visual assessment for detecting apnea in patients undergoing a long procedure with deep sedation [23]. The physical appearance of the propofol made this study difficult to blind.
Conclusion
Our study demonstrated that administration of propofol in combination with fentanyl or dexmedetomidine rather than as a single agent to ERCP patients ensured effective and reliable sedation, reduced total dose of propofol, increased endoscopist satisfaction, decreased the pain level, and ensured haemodynamic stabilization. We consider dexmedetomidine as the most appropriate adjuvant agent because it reduces the pain level and the amount of propofol to be administered to the greatest extent and is not different from other agents in terms of side effects.
Data are presented as either mean values±SD or by absolute numbers.
* Analysed with the ANOVA test; ** Analysed with the Chi-square test.
Values expressed as mean VAS±standard deviation (SD).
* Analysed with the ANOVA test; **Analysed with the Kruskal-Wallis test.
Data presented in number (%) of patients.