Zygomycetes are a group of filamentous fungi which are ubiquitous in environment and capable of causing infection by inhalation of spores or inoculation of fungi into disrupted skin. These fungi belong to two orders, Mucorales and Entomophthorales. The order Mucorales contains family Mucoraceae, Cunninghamellaceae, Saksenaeaceae and Syncephalastraceae, among others. Entomophthorales includes Basidiobolus species and Conidiobolus species. The genera which cause infections are Mucor, Absidia, Rhizopus, Rhizomucor, Apophysomyces, Saksenaea, Cunninghamella, Cokeromyces and Syncephalostrum. Rhizopus is the most common cause of zygomycosis followed by Mucor, Absidia and Rhizomucor [1,2].
Zygomycosis, a fungal infection caused by Zygomycetes, ranges in severity from mild cutaneous infection to highly invasive and disseminated disease. It usually occurs in rhinocerebral, pulmonary, abdominal, pelvic, cutaneous, subcutaneous, disseminated forms. Zygomycosis constitutes the third most common invasive fungal infection following aspergillosis and candidiasis. The infections are more severe in immunocompromised than immunocompetent hosts [3].
Cutaneous zygomycosis usually occurs as gradual and slowly progressive disease which recovers completely with proper diagnosis and treatment but may sometimes become fulminant leading to necrotizing lesions and haematogenous dissemination [4].
Materials and Methods
The present retrospective study was carried out at the Department of Microbiology, Seth GS Medical College and KEM Hospital, Mumbai, Maharashtra, India. All the diagnosed cases of cutaneous zygomycosis caused by Saksenaea vasiformis during the period from July 2010 to June 2017 were included in the study.
The detailed case history of patients with respect to site of involvement, underlying disease, clinical course, agents identified, treatment given and outcome of disease was recorded. For Laboratory diagnosis of zygomycosis, specimens were subjected to 10% KOH examination, culture on SDA, identification of fungi by slide culture and Water Agar method [8]. Specimens processed in the laboratory were tissue biopsies, pus and exudates. A 10% KOH mount of each specimen was prepared and examined under microscope.
Culture on SDA: Specimen inoculated on SDA slants and incubated at 30°C in BOD incubator. Culture was examined daily for characteristic fungal growth for at least a week.
Water agar floatation method for sporulation: Agar block inoculated with fungal growth was floated in sterile distilled water in a petri dish and incubated. Mycelial growth was observed extending from agar block into distilled water after five days [8].
Results
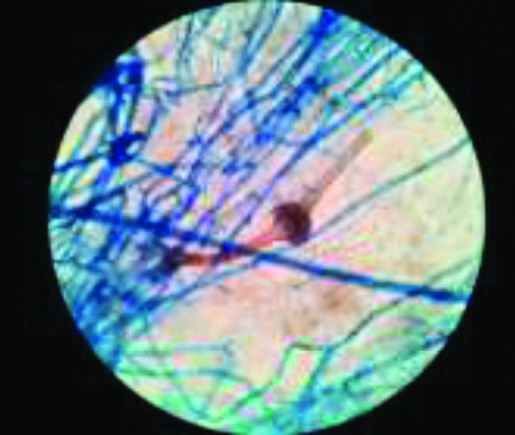
Ten percentage KOH examination showed broad, aseptate fungal hyphae [Table/Fig-1]. Fungus grew as white cottony growth appeared after 48 hours on SDA [Table/Fig-2]. Diagnosis of Saksenaea vasiformis was established on the basis LPCB mount made from growth on water agar plate which showed broad, ribbon shaped aseptate hyphae with a short sporangiophore bearing classical flask shaped sporangia and darkly pigmented rhizoids [Table/Fig-3]. [Table/Fig-4] shows clinical details of the cases.
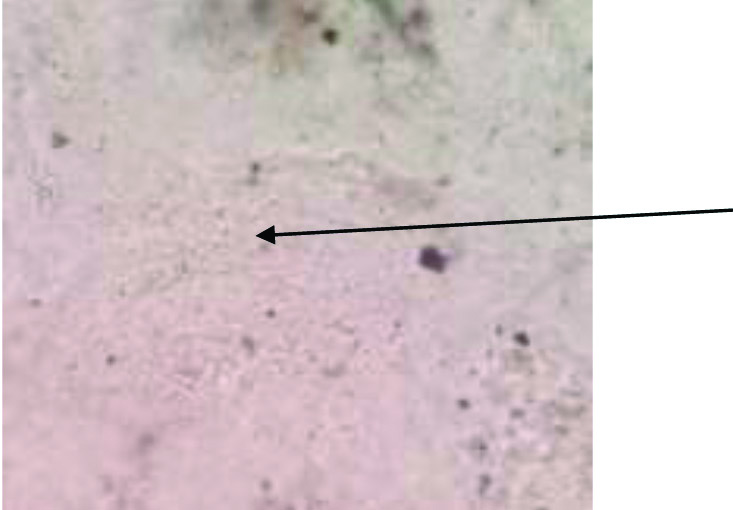
10% KOH examination showing broad, aseptate fungal hyphae (40X) (indicated with arrow).
White cottony growth of zygomycetes on SDA.

LPCB mount showing flask shaped sporangium on short sporangiophores in water agar method characteristic of Saksenaea vasiformis (40x).
Clinical details of the cases.
| Case No. | Age/Gender | Clinical presentation | H/O of trauma/ Surgery | Underlying condition | Management | Outcome |
|---|
| 1. | 23/F | Subcutaneous, indurated, tender swelling over left arm which lead to a nonhealing ulcer, measuring 7×4 cm, after debridement | No | None | Debridement and Amphotericin B 0.5 to 0.75 mg/kg | Cured |
| 2. | 70/F | A painful ulcer measuring 2×3 cm with foul smelling discharge over the right arm. Oedematous, erythematous skin with blackening | H/O Fracture Humerus | Diabetes | Debridement and Amphotericicn B0.5 to 0.75 mg/kg | Died |
| 3. | 30/F | Necrotic wound on abdominal wall following tubal ligation | Post Op Wound | No | Debridement and Amphotericicn B 0.5 to 0.75 mg/kg plus posaconazole 400 mg BID daily | Died |
| 4. | 45/M | Multiple ulcers over arm, abdominal wall, cheek, back with blackish discoloration and discharging pus, intermittent fever | No | Diabetes | Debridement | Died |
| 5. | 21/F | Necrotic wound at laproscopic port site leading to cellulitis and septic shock | H/O Diagnostic Laproscopy | No | Debridement and Amphotericin B0.5 to 0.75 mg/kg | Died |
| 6. | 45/M | Necritic ulcer on chest | No | No | Debridement and Amphotericin B | Cured |
| 7. | 27/F | Traumatic wound on thigh | Yes | No | Debridement and Amphotericin B0.5 to 0.75 mg/kg | Died |
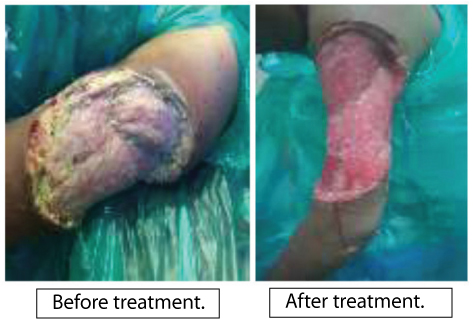
Case 1: A female patient 23-year-old, presented with an indurated, tender swelling over the left arm for 3 months, which lead to an ulcer. She had used many antibiotics but the ulcer was not healing. She was non diabetic, afebrile. The slough excision was done and bits of excised skin and subcutaneous tissue were processed by KOH, LPCB and culture for fungus was put in SDA. KOH & LPCB mount showed broad, aseptate fungal hyphae. SDA after 48 hours showed white cottony growth. LPCB mount from this growth showed only broad aseptate hyphae, no sporulation was seen. Subculutre on corn meal agar also failed to accomplish sporulation. To induce sporulation subculture was made on water agar. Agar block technique was adopted to achieve sporulation. Mycelial growth was observed extending from agar blocks into distilled water on 5th day. LPCB mount from this growth showed broad aseptate ribbon like hyphae characteristic of Zygomycetes and typical flask shaped sporangium with short sporangiophore and darkly pigmented rhizoids [Table/Fig-3]. The diagnosis of Saksenaea vasiformis was confirmed depending on typical morphology. Repeated surgical debridement was done and amphotericin B was administered. The patient recovered and was completely cured.
Case 2: A 70-year-old female patient known diabetic for 10 years presented with a history of fracture of left humerus one month back, and had undergone treatment for the same. She presented with a painful ulcer with foul smelling discharge over the left arm [Table/Fig-5]. On examination, it an oedematous, white cottony growth was seen at the wound margins. Tissue sample was sent to mycology section, KOH mount was made, which showed classical ribbon shaped, broad aseptate hyphae. Subcultre on SDA, after 48 hours it showed white cottony growth. LPCB mount from this growth showed only broad aseptate hyphae, no sporulation was seen. Subculutre on corn meal agar also failed to accomplish sporulation. To induce sporulation subculture was made on water agar. The fungal culture confirmed the diagnosis of Saksenaea vasiformis, patient was started on amphotericin B and surgical debridement was done. Patient didn’t respond to treatment and died on day 12 of admission.
A case of trauma over left arm which led to a non healing ulcer (case 2).
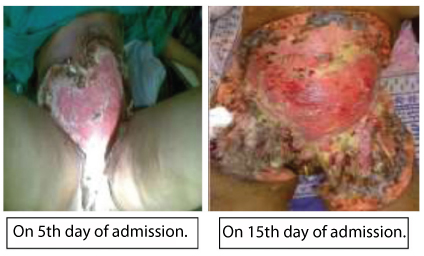
Case 3: A 30-year-old immunocompetent female was admitted, and presented with a necrotic wound on abdominal wall following tubal ligation 15 days ago [Table/Fig-6]. The wound was spreading rapidly and bacterial culture was sterile. On examination blackening of the wound margins was noted. Surgical debridement was done and tissue sent for fungal culture. KOH mount was made, subculture done on SDA and cornmeal agar. LPCB mount from the growth on culture showed only broad aseptate hyphae, no sporulation was seen. To induce sporulation subculture was made on water agar. The diagnosis of Saksenaea vasiformis was confirmed only after the LPCB mount from the growth on wateragar showed broad aseptate ribbon like hyphae characteristic of Zygomycetes and typical flask shaped sporangium with short sporangiophore and darkly pigmented rhizoids. Repeated surgical debridement was done. Amphotericin B 0.5mg per kg body weight was started, after 1 week posaconazol 400 mg BD daily was added but the lesion did not heal, eventually patient succumbed on 25th day of admission.
Postoperative abdominal wound following tubal ligation showing necrosis of anterior abdominal wall (Case 3).
Case 4: A 45-year-old male known diabetic from 15 years presented with multiple ulcers over arm, abdominal wall, cheek and back, with blackish discolouration and discharging pus with intermittent fever. There was no history of trauma or surgery. He had used many antibiotics but the ulcer was not healing. His blood sugar was elevated 450 mg/dL, and was receiving insulin injections but blood sugar was not being controlled. Surgical debridement was done. Necrosed tissue was sent for fungal culture. He died on 5th day of admission. Diagnosis of Saksenaea vasiformis was confirmed postmortem on basis of microscopic and macroscopic characteristics of growth from patient’s debrided tissue sample.
Case 5: A 21-year-old female developed a necrotic wound at laparoscopic port site, leading to cellulitis and septic shock. Antibiotics were started and amphotericin B 0.5 mg per kg body weight was added on day eight. Surgical debridement was done and tissue sent for fungal culture. KOH mount was made, subculture done on SDA and cornmeal agar. LPCB mount from the growth on culture showed only broad aseptate hyphae, no sporulation was seen. To induce sporulation subculture was made on water agar. The diagnosis of Saksenaea vasiformis was confirmed only after the LPCB mount from the growth on water agar showed broad aseptate ribbon like hyphae characteristic of Zygomycetes and typical flask shaped sporangium with short sporangiophore and darkly pigmented rhizoids. She didn’t respond to treatment and died on day 18 of admission
Case 6: A 45-year-old male, non-diabetic had a non-healing, necrotic ulcer on chest. He gave a history of minor trauma at the same site due to ECG electrode. Diagnosis of Saksenaea vasiformis was confirmed after the fungal culture report. Surgical debridement was done and amphotericin B administered. The lesion was cured completely.
Case 7: A 27-year-old immunocompetent female, had a traumatic wound on right thigh. On examination white cottony growth was seen around the margins. Detailed history of the patient revealed that the wound was contaminated with soil. She didn’t seek any medical advice for 2 weeks. When she came to hospital, wound debridement was done, Amphotericin B started but she died within 72 hours of admission, diagnosis of Saksenaea vasiformis was confirmed postmortem on basis of microscopic and macroscopic characteristics of growth from patient’s debrided tissue sample.
Discussion
Zygomycosis was described as a cause of human diseases in 1885 [3] and the first cutaneous case was reported in 1929. Incidence of invasive zygomycosis has been increasing over past two decades with increased prevalence in diabetics and immunocompromised patients. Rhizopus, Mucor, Rhizomucor, Absidia are the common causative organisms while Saksenaea vasiformis, Apophysomyces elegans, Basidiobolus ranarum are rare causes [9-11].
Cutaneous zygomycosis is the third most common form of zygomycosis (19%) following rhinocerebral (39%) and pulmonary disease (24%). Unlike other presentations of zygomycosis, 40-50% of patients with cutaneous zygomycosis are immunocompetent [4,12].
Cutaneous zygomycosis can present as localized, deep or disseminated disease. Almost 50% of patients with cutaneous zygomycosis have their disease confined to cutaneous and subcutaneous tissue, while in 24% cases disease may extend to bone, tendon, muscle, and deeper tissues. Upper & lower extremities are the common sites of involvement, although any area of skin can be affected [13].
Cutaneous and subcutaneous infection by Saksenaea vasiformis usually present as an erythematous swelling. It may lead to painful ulcerative lesion with or without discharging pus, necrotic tissue and fever. Sometimes, the superficial infection can lead to invasive and disseminated infection resulting in multisystem involvement and sepsis [12,14].
Angoinvasive nature of the Saksenaea vasiformis accounts for the severity of disease both in immunocompetent and immunocompromised patients. Local invasion resulting in necrotizing soft tissue may progress to necrotizing fasciitis and/or gangrene in large number of cases [14,15].
The fungi causing zygomycosis are not able to enter through the intact skin. The predisposing factor for infections is the break in the normal protective cutaneous barrier.
Direct inoculation in skin after penetrating trauma, burns, motor vehicle accident, are major routes of entry of agents. Surgery, surgical splints, catheter lines, biopsy sites, injection sites, tattoos can also become portal of entry of organisms. The insect bites can also lead to this infection [10,16].
Zygomycosis can also be a nosocomial infection as it has been associated with surgical procedures, medication patches, intravenous catheters [3].
Hyperglycaemia, ketoacidosis, haematological or any malignancy, leucopenia, immunosuppressive therapy, organ transplant, chronic renal failure are the systemic risk factors [14,17]. Factors predisposing diabetics to zygomycosis are impaired chemotaxis and defective killing by phagocytes, acidic pH and increased level of iron facilitating the growth of zygomycetes, increased expression of Glucose Regulated Protein (GRP-78) mediating penetration and damage of endothelial cells by zygomycetes leading to angioinvasion [18,19].
In our study, majority of patients were immunocompetent and presented with necrotic non healing ulcer with foul smelling discharge. The patient did not respond to antibiotics. History of diabetics, trauma and postoperative wound infection was presented by two patients each. Inspite of extensive surgical debridement and use of antifungals, five patients scummed to infection and only two patients survived.
A case study on cutaneous zygomycosis by Saksenaea vasiformis in an immunocompetent host where in patient developed ulcer at the site of nodule on cheek. The wound was debrided and amphotericin B administered which cured the patient [12].
A case study on necrotizing fascitis by Saksenaea vasiformis, a 35-year-old male developed infection at the site of surgical incision, after 4-5 days of surgery. Inflammation, oedema, white cottony growth coming out of the wound gape was observed. The patient was suffering from interstitial nephritis, therefore the patient was started on 50% dose of Amphotericin B and emergency debridement was planned as soon as the diagnosis was confirmed. But the patient didn’t comply with and got discharged against medical advice, later he expired [15].
A study on primary cutaneous zygomycosis by Saksenaea vasiformis and Apophymsomyceselegan, one of the two cases, the patient developed infection at the site of surgical incision. Swelling and blackish discolouration developed at the site of infection. Surgical debridement of the wound was done and antifungals were given, but the patient did not survive. The infection was diagnosed at postmortem [14].
In a case report of a 49-year-old diabetic female, she had an arthropod bite on the arm. She failed to respond to antibiotics. There was discoloration and erythema over the triceps area at admission. Later it developed into an ulcer. After the diagnosis of Saksenaea vasiformis was confirmed, Amphotericin B was administered and surgical debridement was done. The infection was controlled with surgical debridement, amphotericin B and normalization of blood sugar. Although disease due to Saksenaea vasiformis is strongly associated with soil contamination of traumatic wound, this patient suffered minimal trauma and soil contamination of the wound was not recognized. Hyperglycaemia may have contributed to establishment and progression of disease [18].
In a case study done on cutaneous zygomycosis by Saksenaea vasiformis in a tertiary care centre in north-west India, total 9 cases of cutaneous zygomycosis were diagnosed. Out of those, in one of the cases, 60-year-old female, Saksenaea vasiformis was identified. The necrotic lesion had formed on the gluteal region at the site of IM injection. Local debridement was done and Amphotericin B administered but patient died due to bacterial sepsis [2].
To increase the survival chances of patient’s prompt diagnosis with appropriate treatment is required. High degree of suspicion is required for diagnosis of zygomycosis. Non-healing wound not responding to antibacterial, blackish discoloration, history of trauma, surgery in recent past, contamination with soil should raise the suspicion of zygomycosis [18,20].
Treatment of zygomycosis consists of repeated surgical debridement and antifungal drugs. Surgery decreases the fungal load in the lesions and also facilitates entry of antifungals in cases of massive tissue necrosis. Lipid formulation of amphotericin B is the treatment of choice. Posaconazole is a good alternative in patients with amphotericin induced toxicity. Hyperbaric oxygen therapy can be been used as an adjuvant therapy [1,16].
Treatment should always be continued along with reversal of underlying risk factor. Rapid control of underlying diabetes, tapering of steroid therapy usually adds to positive outcome of therapy [1,17].
After the inoculation, Saksenaea vasiformis invade blood vessels causing thrombosis which leads to ischemic tissue necrosis. Rapid progression to necrosis typically within days along with rapid growth of organism in the blood vessels usually contributes to the poor outcome of therapy. Reduction of environmental exposure to pathogen, although not easily feasible is the main prophylactic measure. Iatrogenic risk factors are controlled by using proper aseptic technique [18].
Limitation
Being a retrospective study, this study has the limitation of analysing cases already documented in our department. Being a rare pathogen, the number of cases is small and further study is warranted to establish role of this unusal pathogen Saksenaea vasiformis.
Conclusion
Saksenaea vasiformis infection is one of the differential diagnosis in cases of rapidly progressive necrosis of wound not responding to antibiotics. A high level of clinical suspicion coupled with laboratory confirmation is must for correct diagnosis and proper management of infection.