Introduction
The HCV infection is an emerging condition in ESRD patients on maintenance HD, which constitutes a major burden complicating the dialysis process in dialysis units [1]. The worldwide prevalence of HCV on maintenance HD patients varies between 1-85% [2]. In developing countries like India, the prevalence of HCV is particularly high and is a major cause of significant morbidity and mortality in ESRD patients [2]. Morbidity and mortality is significantly higher in HCV infected dialysis patients and these patients succumb even before the long-term HCV complications develop [3]. Malnutrition–Inflammation Complex Syndrome (MICS), a condition associated with poor short-term clinical outcome, mortality, increased hospitalisation rate, significant protein-energy malnutrition and decreased health-related quality of life is a concomitant condition in HD patients [4]. Even though HCV is a blood-borne pathogen, several review articles have concluded that transmission may occur due to contaminated dialysers, HD machines, hands of staff members or other shared patient items [5,6]. HCV infected patients on HD have MICS associated metabolic abnormalities such as insulin resistance, oxidative stress, inflammation, and endothelial dysfunction [7]. Earlier studies have reported an association of these abnormalities in HCV infected patients on HD, but this has not been evaluated [8]. Recently, the role of inflammation in the pathogenesis of atherosclerosis has been clearly defined in various studies [4,9]. It has also been shown that HCV seropositivity, carotid artery plaque formation and increased carotid intima-media wall thickness are independently correlated in general population [10]. Some observational studies have shown that hyperhomocysteinemia is an independent risk factor for cardiovascular disease in the general population [8,11]. An effect of hyperhomocysteinaemia on renal failure is related to increased cardiovascular risk. Therefore, with progression of kidney disease, a series of abnormalities develop such as increased oxidative stress, worsening inflammation, and increased serum Hcy levels. To better characterise the impact of HCV on short term cardiovascular mortality, the present study aimed to estimate the levels of MDA, FRAP, hs-CRP, Hcy, NO and CIMT in ESRD patients with and without HCV infection on maintenance HD and compared with the controls.
Materials and Methods
The cross-sectional prospective study was conducted at the Department of Nephrology in collaboration with Department of Biochemistry at Sri Venkateswara Institute of Medical Sciences, Tirupati, Andhra Pradesh, India, for a duration of one year from March 2010 to March 2011. Nineteen healthy controls were included as Group 1 in the present study. The exclusion criteria for controls was chronic kidney disease, Acute Renal Failure (ARF), HCV seropositive status, smoking, paediatric age group (<18 years), pregnant women, unwilling patients, diabetes mellitus, vasculitis, liver disease, malignancy, history of alcohol abuse. The 44 patients with ESRD receiving regular maintenance haemodialysis were recruited in the present study. Among 44 patients with ESRD on maintenance HD, 22 patients with ESRD with seronegative status for HCV were included as Group 2 and 22 patients with ESRD with seropositive status for HCV were included as Group 3.
Exclusion criteria: Patients with positive status for anti-HCV antibodies at the beginning of the HD, ARF, acute on chronic kidney disease, smoking, paediatric age group (<18 years), pregnant women, unwilling patients, vasculitis, liver disease, malignancy, history of alcohol abuse.
The study protocol was approved by the Institutional Ethics Committee. A written informed consent was taken from all the subjects of the study.
Laboratory Analysis
A 5 mL of venous blood sample was drawn in additive free tubes from the end of the dialyser just before commencement of the HD session from both the case groups and 5 mL of fasting venous blood sample was drawn in additive free tubes from the controls. The blood samples were allowed to stand for 30 minutes at room temperature for the retraction of clot, centrifuged (Remi 8RC centrifuge) at 3000 rpm for 15 minutes to separate the serum. The separated serum was stored at -80°C until further analysis.
Serum urea, creatinine were estimated by timed fixed endpoint method and Modified Jaffe’s rate kinetic method by Beckman system pack [12]. Serum hs-CRP was estimated by immunoturbidimetry method using the commercial kit obtained from Tulip Diagnostics Limited-Quantia-CRP [13]. The test sample is mixed with latex reagent and activation buffer. Presence of CRP in the sample results in the formation of an insoluble complex producing a turbidity, which is measured at wavelength between 505-578 nm. Serum Hcy was estimated by enzymatic recyclic method by commercial kit from Bio-Rad, India. The principle of the assay was that homocysteine in the sample was converted to S-adenosyl-L-homocysteine (SAH) in the presence of SAH hydrolase. After the removal of unbound anti-SAH antibody, Horse Radish Peroxidise (HRP) was added. After the addition of substrate, the peroxidase activity in the sample was measured spectrophotometrically and the absorbance was inversely proportional to the concentration of Hcy in the sample [14]. All the above parameters were estimated on Beckman Synchron CX9 Clinical Chemistry autoanalyser, USA. MDA and FRAP were estimated by spectrophotometric method using Perkin-Elmer spectrophotometer (Lambda 25 UV/VIS Spectrophotometer, USA) [15,16]. Serum NO was estimated by Griess method using Perkin-Elmer spectrophotometer (Lambda 25 UV/VIS Spectrophotometer, USA) [17].
CIMT: Carotid Ultrasonography: Both the carotid arteries were investigated ultrasonographically with an AlokaPro sound SSD-5000 using 7.5 Hz high resolution linear probe for Intima Media Thickness (IMT). IMT was measured on the longitudinal views of the far wall of the distal segment of common carotid artery, carotid bifurcation and intimal tract of internal carotid artery on both sides. Measurements performed were 0.5, 1 and 2 cm below and above the bifurcation (six measurements on each side) in a plaque free arterial segment. Each measurement was an average of three measurements by single observer.
Statistical Analysis
All continuous variables were tested for normal distribution with the Kolmogorov–Smirnov test. Normally distributed data were presented as mean±standard deviation. Categorical data were presented as numbers and percent. Comparisons between the groups were analysed by using One-way ANOVA followed by post-hoc analysis. Pearson’s rank correlation was used to determine correlations of CIMT levels with other variables. All statistical analyses were performed with SPSS (version 16.0, SPSS Inc., Chicago, IL, USA). A p-value of <0.05 was considered significant.
Results
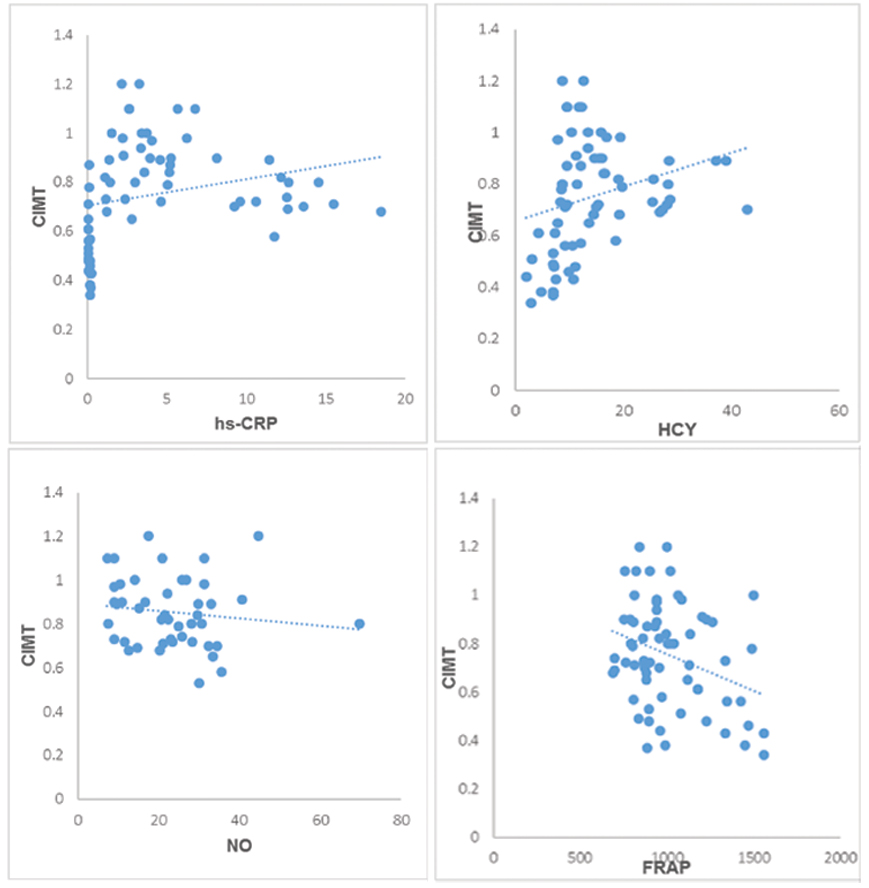
The general demographic characteristics of 22 ESRD patients with HCV positive on HD and 22 ESRD patients with HCV negative on HypertensionHD patients are shown in the [Table/Fig-1]. The mean±SD of the biochemical parameters in ESRD patients of controls and both groups by using One-way ANOVA are shown in the [Table/Fig-2]. The inflammatory marker hs-CRP was found to be significantly elevated in Group 2 and 3 compared with controls (p-value <0.001). The serum MDA was measured as marker of oxidative stress and found to be significantly elevated in Group 2 and 3 compared to controls (p-value<0.001). The serum Hcy levels were found to be increased in Group 2 and 3 compared with controls which was stastically significant (p-value<0.001). The CIMT levels were found to be significantly elevated in Group 2 and 3 compared with controls which was stastically significant (p-value<0.001). The post-hoc analysis of the biochemical parameters studied among three groups was shown in [Table/Fig-3]. In the present study, CIMT is correlated with the other markers and shown in [Table/Fig-4]. The scatter plots showing association of CIMT with hs-CRP, HCY, NO and FRAP [Table/Fig-5].
Showing the Demographic characteristics in different studied groups.
| General characteristics | Group 1 | Group 2 | Group 3 |
|---|
| Age Mean±SD (years) | 52.66±6.33 | 49.36±11.36 | 50.26±12.69 |
| Sample size (n) | 19 | 22 | 22 |
| Gender (Male/Female) | 14/5 | 16/6 | 16/6 |
| DM | - | 9 | 6 |
| HTN | - | 22 | 22 |
| Average duration on HD supports | - | 3.18 years | 4.65 years |
roup 1: control group, Group 2:ESRD with HCV Negative, Group 3: ESRD with HCV Positive,
DM-Diabetes mellitus, HTN-HypertensionHD
Mean±SD of the various biochemical parameters across the groups of the present study.
| Parameter | Group 1 Mean±SD | Group 2 Mean±SD | Group 3 Mean±SD | p-value |
|---|
| MDA (μmol/L) | 2.12±0.82 | 3.08±0.67 | 5.81±0.91 | p<0.001* |
| FRAP (mmol/L) | 1170.45±252.37 | 997.52±191.80 | 884.86±132.51 | p<0.001* |
| Serum NO (μmol/L) | 29.73±7.31 | 18.75±10.83 | 27.45±11.81 | p=0.002* |
| Hcy (μmol/L) | 7.51±2.76 | 12.46±3.26 | 23.12±9.11 | p<0.001* |
| hs-CRP (mg/dL) | 0.1200±0.06 | 3.85±1.77 | 8.66±5.41 | p<0.001* |
| CIMT | 0.52±0.14 | 0.75±0.08 | 0.96±0.13 | p<0.001* |
Group 1: control group, Group 2: ESRD with HCV Negative, Group 3: ESRD with HCV Positive.
Data is expressed as mean±SD. MDA- malondialdehyde, hsCRP-High sensitivity C-reactive Protein, FRAP- Ferric reducing ability of plasma, NO-Nitric oxide. Hcy-Homocysteine
*Significant p-value at the 0.05. (Using One-way ANOVA)
The significance of changes between the groups of the present study.
| Parameter | Group 1 vs. 2 | Group 1 vs. 3 | Group 2 vs. 3 |
|---|
| MDA (μmol/L) | p=0.001* | p<0.001* | p<0.001* |
| FRAP (mmol/L) | p=0.016* | p<0.001* | p=0.085† |
| Serum NO (μmol/L) | p=0.002* | p=0.536† | p=0.018* |
| Hcy (μmol/L) | p=0.019* | p<0.001* | p<0.001* |
| hs-CRP (mg/dL) | p=0.001* | p<0.001* | p=0.002* |
| CIMT | p<0.001* | p<0.001* | p<0.001* |
Group 1: control group, Group 2:ESRD with HCV Negative, Group 3: ESRD with HCV Positive.
MDA-malondialdehyde, hsCRP-High sensitivity C-reactive Protein, FRAP-Ferric reducing ability of plasma, NO-Nitric oxide. Hcy-Homocysteine
*Significant p-value at the 0.05. †NS, Not significant at the 0.05 probability level. (Using Post-Hoc analysis- Bonferroni test)
Table showing the correlation of CIMT with biochemical markers of the present study.
| Parameter | r | p-value |
|---|
| MDA | 0.157 | 0.208† |
| FRAP | -0.322 | 0.008** |
| NO | -0.268 | 0.030* |
| Hcy | 0.268 | 0.029* |
| hs-CRP | 0.259 | 0.049* |
MDA- Malondialdehyde, hsCRP-High sensitivity C-reactive protein, FRAP-Ferric reducing ability of plasma, NO-Nitric oxide. Hcy-Homocysteine. r-correlation coefficient
**Significant at the 0.01 probability level *Significant at the 0.05 probability level, †NS, Not significant at the 0.05 probability level (Using Pearson correlation coefficient)
Scatter plots showing association of CIMT with hs-CRP, HCY, NO and FRAP.
X-axis- independent variables and Y-Axis-dependent variable (CIMT)
Discussion
Chronic inflammation and oxidative stress are highly prevalent in patients with chronic kidney disease and ESRD. The gold standard among the microinflammatory markers for the measurement of inflammation is CRP [11]. In the present study the effect of HCV infection in patients on maintenance HD with state of inflammation by comparing the values of hsCRP between those with and without HCV infection was analysed. Increased levels of serum hs-CRP were found in ESRD patients with and without HCV infection on maintenance HD as compared to normal controls which were found to be statistically significant (p-value <0.001). Comparative analysis between the groups with HCV positive and negative status also yield significantly high levels of hs-CRP in HCV positives than HCV negatives. Several factors contribute to inflammation in patients on maintenance HD [18]. They are classified into patient related and dialysis technique related. Patient related factors include underlying disease, comorbidities, oxidative stress, increased calcium phosphorous product, occult infections like periodontitis and vascular access infections, anaemia, immunologic factors and sedentary lifestyle. Dialysis technique related factors include retention of inflammatory mediators, oxidative imbalance, acetate, pyrogenic substances of the dialysate and complement activation due to the use of certain bio incompatible materials [8]. The central roles in MICS might be played by pro-inflammatory cytokines generated during HD as evidenced by increased hs CRP, Interleukin-6 (IL-6) and TNF alpha levels in HCV-infected patients on Maintenance Haemodialysis (MHD) reported by Tsai HB et al., [19]. All of the above findings agree with the results of the present study. In the present study, increased oxidative stress was noted in ESRD patients on MHD with hepatitis c positive status compared to hepatitis c negative status. MDA has been taken as the representative of oxidative injury and FRAP has been chosen to suggest the antioxidative status. In the present study, statistically significant increased MDA levels in both HCV positive and negative patients on maintenance haemodialysis were observed as compared to controls, suggesting increased oxidative stress (p-value <0.001). In the present study, the comparative analysis between HCV positive and negative patients in terms of MDA levels observed statistically significant increased MDA levels in HCV infected patients on MHD as compared to non-infected patients on MHD. This is in concurrence with the existing observations in the literature who considered oxidative stress as a potentially important source of patient morbidity and mortality in HCV infected patients on MHD [20]. In HCV infected patients on MHD, the infection stimulates the production of Reactive Oxygen Species (ROS) by activated macrophages and hepatocytes. Liver tissue and plasma samples from infected patients show an increase in the lipid peroxidation products. HCV replication is found to disturb Endoplasmic Reticulum (ER) inducing ER stress which stimulates protein degradation and depletes ER calcium stores, a factor that stimulates increased oxidative stress [21]. In addition, the viral core protein also found to increase oxidative stress through its action on the mitochondria [22]. With regard to antioxidant activity demonstrated by FRAP assay the present study in did not found any statistically significant reduction in HCV infected patients as compared to non-infected HCV patients. However, the antioxidant activity was significantly low in patients with ESRD on MHD as compared to controls which in turn suggests its utilization in mitigating oxidative stress as compared to controls.
The ESRD results in tenfold higher cardiovascular mortality rate [23]. Endothelial dysfunction is a potential mechanism that contributes to cardiovascular risk and is characterized by reduced synthesis or bioavailability of NO [24]. In the present study, serum nitrate levels had been taken as an indicator of NO production. NO levels were found to be low in patients receiving HD as compared to controls as shown in [Table/Fig-2]. The amount of NO produced is dependent upon substrate (arginine) availability and the presence of inhibitors of Nitric Oxide Synthase (NOS) and Asymmetric Dimethylarginine (ADMA). Plasma nitrate levels were found to be elevated in patients on MHD with HCV infection than without infection. Several studies had showed increased serum homocysteine levels to be an independent risk factor in maintenance haemodialysis patients causing atherosclerotic vascular disease, vascular access thrombosis and increased cardiovascular mortality in patients undergoing dialysis therapy [11,25]. In the present study, statistically significant increased serum Hcy levels were found in patients on MHD as compared to controls. In the present study, serum Hcy levels were compared in patients with HCV positive and negative status on MHD and observed statistically significantly higher Hcy levels in those with HCV infection as compared to those without infection (p-value <0.001). In a study by Suliman ME et al., observed that the levels of Hcy were elevated in 95% of the patients on MHD but that the absolute level of Hcy appears to be dependent on nutritional status, intake of protein and serum albumin levels which can be related in terms of cardiovascular risk [26]. The proposed mechanisms by which homocysteine induces atherosclerosis might be enhancement of oxidative stress, modulation of coagulation and fibrinolytic activity, promotion of vascular smooth muscle proliferation and endothelial dysfunction [27]. Haemodialysis patients are known to have an advanced carotid artery intimal medial thickness compared with age and gender matched normal subjects. In the present study, carotid intimal medial thickness was evaluated in patients on MHD as compared to controls. Further the differences in measurements of CIMT in patients on MHD in those with HCV infection and those without were assessed. When compared with controls the patients on MHD had significantly higher CIMT mean values. Hence, the morbidity and mortality of HCV infected HD patients are higher than the non-infected ones. Consistent with these data, other cross-sectional studies from both Western and Asian populations reported a higher prevalence of carotid atherosclerosis in individuals infected with HCV compared to non-infected controls [28,29]. It has been demonstrated that HCV colonises and replicates within carotid plaques likely causing vascular inflammation. HCV promote atherogenesis through several direct and indirect mechanisms involving HCV colonisation and replication within arterial walls, enhanced and imbalanced secretion of inflammatory cytokines, oxidative stress, endotoxemia, mixed cryoglobulinaemia, perturbed cellular and humoral immunity, hyperhomocysteinaemia, hypo-adiponectinaemia, insulin resistance, type 2 diabetes and other components of the metabolic syndrome [30]. Earlier studies conducted in the general population showed that HCV markers were independently associated with atherosclerosis [30,31,32]. These findings were supported by Zaki MSE, who reported that HCV has a significant effect on the development of cardiovascular diseases in the general population, and in renal disease patients on the structural level [33].
Limitation
The sample size for the control group is less compared to that of test group due to drop outs. Moreover, due to time constraints, the sample size was small and also a difference in the sample size was found in between the groups.
Conclusion
The present study findings summarise the possibility of contribution of HCV infection to oxidative stress, inflammation and endothelial dysfunction in addition to chronic kidney disease and dialysis. The present study findings also support the view that the HCV infection is a risk factor for atherosclerosis and cardiovascular mortality in ESRD patients on maintenance haemodialysis.
roup 1: control group, Group 2:ESRD with HCV Negative, Group 3: ESRD with HCV Positive,DM-Diabetes mellitus, HTN-HypertensionHD
Group 1: control group, Group 2: ESRD with HCV Negative, Group 3: ESRD with HCV Positive.Data is expressed as mean±SD. MDA- malondialdehyde, hsCRP-High sensitivity C-reactive Protein, FRAP- Ferric reducing ability of plasma, NO-Nitric oxide. Hcy-Homocysteine*Significant p-value at the 0.05. (Using One-way ANOVA)
Group 1: control group, Group 2:ESRD with HCV Negative, Group 3: ESRD with HCV Positive.MDA-malondialdehyde, hsCRP-High sensitivity C-reactive Protein, FRAP-Ferric reducing ability of plasma, NO-Nitric oxide. Hcy-Homocysteine*Significant p-value at the 0.05.
†NS, Not significant at the 0.05 probability level. (Using Post-Hoc analysis- Bonferroni test)
MDA- Malondialdehyde, hsCRP-High sensitivity C-reactive protein, FRAP-Ferric reducing ability of plasma, NO-Nitric oxide. Hcy-Homocysteine. r-correlation coefficient**Significant at the 0.01 probability level *Significant at the 0.05 probability level,
†NS, Not significant at the 0.05 probability level (Using Pearson correlation coefficient)
[1]. Liu CH, Liang CC, Liu CJ, Lin JW, Chen SI, Hung PH, Pegylated interferon alfa-2a monotherapy for hemodialysis patients with acute hepatitis CClin Infect Dis 2010 51(5):541-59.10.1086/65568220645865 [Google Scholar] [CrossRef] [PubMed]
[2]. Chigurupati P, Subbarayudu S, Babu S, Study of incidence of hepatitis C virus infection in hemodialysis patientsAnn Trop Med Public Health 2014 7:167-70.10.4103/1755-6783.149499 [Google Scholar] [CrossRef]
[3]. Liu CH, Kao JH, Treatment of hepatitis C virus infection in patients with end-stage renal diseaseJ Gastroenterol Hepatol 2011 26(2):228-39.10.1111/j.1440-1746.2010.06488.x21261711 [Google Scholar] [CrossRef] [PubMed]
[4]. Ho LC, Wang HH, Peng YS, Chiang CK, Huang JW, Hung KY, Clinical utility of malnutrition inflammation score in maintenance hemodialysis patients: focus on identifying the best cut-off pointAm J Nephrol 2008 28(5):840-26.10.1159/00013768418535370 [Google Scholar] [CrossRef] [PubMed]
[5]. Martin P, Fabrizi F, Hepatitis C virus and kidney diseaseJ Hepatol 2008 49(4):613-24.10.1016/j.jhep.2008.06.00318662838 [Google Scholar] [CrossRef] [PubMed]
[6]. Lindley EJ, Boyle G, Gandy D, Hardy A, Harrington M, Hoenich NA, How plausible is transmission of hepatitis C virus via the haemodialysis circuit?NDT Plus 2011 4:434-36.10.1093/ndtplus/sfr10025984217 [Google Scholar] [CrossRef] [PubMed]
[7]. Morena M, Canaud B, Terrier N, Ludovic C, Cristol JP, Oxidative stress complex syndrome: The dark side of the malnutrition-inflammation complex syndromeHemodial Int 2007 11(1):S32-38.10.1111/j.1542-4758.2007.00144.x [Google Scholar] [CrossRef]
[8]. Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Miller LG, Daar ES, Gjertson DW, Hepatitis C virus and death risk in hemodialysis patientsJ Am Soc Nephrol 2007 18(5):1584-93.10.1681/ASN.200607073617429053 [Google Scholar] [CrossRef] [PubMed]
[9]. Jofré R, Rodriguez-Benitez P, López-Gómez JM, Pérez-Garcia R, Inflammatory syndrome in patients on hemodialysisJ Am Soc Nephrol 2006 17(1):274-80.10.1681/ASN.200608092617130274 [Google Scholar] [CrossRef] [PubMed]
[10]. Fukui M, Kitagawa Y, Nakamura N, Yoshikawa T, Hepatitis C virus and atherosclerosis in patients with type 2 diabetesJAMA 2003 289(10):1245-46.10.1001/jama.289.10.1245-b12633185 [Google Scholar] [CrossRef] [PubMed]
[11]. Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH, A malnutrition-inflammation score iscorrelated with morbidity and mortality in maintenance haemodialysis patientsAm J Nephrol 2001 38(6):1251-63.10.1053/ajkd.2001.2922211728958 [Google Scholar] [CrossRef] [PubMed]
[12]. Vaishya R, Arora S, Singh B, Mallika V, Modification of jaffe’s kinetic method decreases bilirubin interference: A preliminary reportIndian J Clin Biochem 2010 25(1):64-66.10.1007/s12291-010-0013-223105886 [Google Scholar] [CrossRef] [PubMed]
[13]. Sahoo RC, Acharya PR, Noushad TH, Anand R, Acharya VK, Sahu KR, A study of high-sensitivity c-reactive protein in bronchial asthmaIndian J Chest Dis Allied Sci 2009 51(4):213-16. [Google Scholar]
[14]. Ueland PM, Refsum H, Stabler SP, Malinow MR, Andersson A, Aleen RH, Total homocysteine in plasma or serum: Methods and clinical applicationsClin chem 1993 39(9):1764-79. [Google Scholar]
[15]. Ohkawa H, Ohishi N, Yagi K, Assay for lipid peroxides in animal tissues by thiobarbituric acid reactionAnal Biochem 1979 95:351-58.10.1016/0003-2697(79)90738-3 [Google Scholar] [CrossRef]
[16]. Benzie IF, Strain JJ, The ferric reducing ability of plasma as a measure of “antioxidant power”: the FRAP assayAnal Biochem 1996 239:70-76.10.1006/abio.1996.02928660627 [Google Scholar] [CrossRef] [PubMed]
[17]. Moshage B, Kok H, Huizenga JR, Jansen PL, Nitrite and nitrate determination in plasma: A critical evaluationClin Chem 1995 41:892-96. [Google Scholar]
[18]. Caliskan Y, Oflaz H, Pusuroglu H, Boz H, Yazici H, Tamer S, Hepatitis C virus infection in hemodialysis patients is not associated with insulin resistance, inflammation and atherosclerosisClinical Nephrology 2009 71(2):147-57.10.5414/CNP7114719203507 [Google Scholar] [CrossRef] [PubMed]
[19]. Tsai HB, Chen PC, Liu CH, Hung PH, Chen MT, Chiang CK, Association of hepatitis C virus infection and malnutrition-inflammation complex syndrome in maintenance hemodialysis patientsNephrol Dial Transplant 2012 27(3):1176-83.10.1093/ndt/gfr45821896499 [Google Scholar] [CrossRef] [PubMed]
[20]. Locatelli F, Canaud B, Eckardt KU, Stenvinkel P, Wanner C, Zoccali C, Oxidative stress in end-stage renal disease: an emerging threat to patient outcomeNephrol Dial Transplant 2003 18(7):1272-80.10.1093/ndt/gfg07412808161 [Google Scholar] [CrossRef] [PubMed]
[21]. Tardif KD, Waris G, Siddiqui A, Hepatitis C virus, ER stress, and oxidative stressTrends Microbiol 2005 13(4):159-63.10.1016/j.tim.2005.02.00415817385 [Google Scholar] [CrossRef] [PubMed]
[22]. Sezer S, Tutal E, Aldemir D, Turkoglu S, Demirel OU, Afsar B, Hepatitis C infection in hemodialysis patients: protective against oxidative stress?Transplant Proc 2006 38:406-10.10.1016/j.transproceed.2005.12.09416549132 [Google Scholar] [CrossRef] [PubMed]
[23]. Alani H, Tamimi A, Tamimi N, Cardiovascular co-morbidity in chronic kidney disease: Current knowledge and future research needsWorld J Nephrol 2014 3(4):156-68.10.5527/wjn.v3.i4.15625374809 [Google Scholar] [CrossRef] [PubMed]
[24]. Bian K, Murad F, Nitric oxide (NO): biogeneration, regulation, and relevance to human diseasesFront Biosci 2003 8:264-78.10.2741/99712456375 [Google Scholar] [CrossRef] [PubMed]
[25]. Miyazaki H, Matsuoka H, Itabe H, Usui M, Ueda S, Okuda S, Hemodialysis impairs endothelial function via oxidative stressCirculation 2000 101:1002-06.10.1161/01.CIR.101.9.100210704167 [Google Scholar] [CrossRef] [PubMed]
[26]. Suliman ME, Qureshi AR, Barany P, Stenvinkel P, Filho JC, Anderstam B, Hyperhomocysteinemia, nutritional status, and cardiovascular disease in hemodialysis patientsKidney Int 2000 57(4):1727-35.10.1046/j.1523-1755.2000.00018.x10760109 [Google Scholar] [CrossRef] [PubMed]
[27]. Perna AF, Acanfora F, Satta E, Lombardi C, Ingrosso D, De Santo NG, Hyperhomocysteinemia and cardiovascular Disease in Uremia: The newest evidence in epidemiology and mechanisms of actionSemin Nephrol 2004 24:426-30.10.1016/j.semnephrol.2004.06.01915490404 [Google Scholar] [CrossRef] [PubMed]
[28]. Kalantar-Zadeh K, McAllister CJ, Miller LG, Clinical characteristics and mortality in hepatitis C-positive hemodialysis patients: A population based studyNephrol Dial Transplant 2005 20(8):1662-69.10.1093/ndt/gfh89515905194 [Google Scholar] [CrossRef] [PubMed]
[29]. Petta S, Hepatitis C virus and cardiovascular: A reviewJ Adv Res 2017 8(2):161–-68.10.1016/j.jare.2016.06.00128149651 [Google Scholar] [CrossRef] [PubMed]
[30]. Adinolfi LE, Zampino R, Restivo L, Lonardo A, Guerrera B, Marrone A, Chronic hepatitis C virus infection and atherosclerosis: Clinical impact and mechanismsWorld J Gastroentrol 2014 20(13):3410-17.10.3748/wjg.v20.i13.341024707124 [Google Scholar] [CrossRef] [PubMed]
[31]. Ishizaka Y, Ishizaka N, Takahashi E, Unuma T, Tooda E, Hashimoto H, Association between Hepatitis C Virus Core Protein and Carotid AtherosclerosisCirc J 2003 67:26-30.10.1253/circj.67.2612520147 [Google Scholar] [CrossRef] [PubMed]
[32]. Boddi M, Abbate R, Chellini B, Giusti B, Giannini C, Pratesi G, Hepatitis C virus RNA localization in human carotid plaquesJ Clin Virol 2010 47(1):72-75.10.1016/j.jcv.2009.10.00519896417 [Google Scholar] [CrossRef] [PubMed]
[33]. Zaki MSE, The effect of Hepatitis C Virus infection on cardiovascular complications in end stage kidney disease patients on regular hemodialysisElectronic Physician 2017 9(2):3857-61.10.19082/385728465818 [Google Scholar] [CrossRef] [PubMed]