Adenocarcinoma of the Sigmoid Colon Following Ureterosigmoidostomy: A Rare Long Term Sequela
Manjunatha Sathyanarayanaprasad1, Suresh Bhat2, Fredrick Paul3
1 Senior Resident, Department of Urology, Government Medical College, Gandhi Nagar, Kottayam, Kerala, India.
2 Professor and Head, Department of Urology, Government Medical College, Gandhi Nagar, Kottayam, Kerala, India.
3 Associate Professor, Department of Urology, Government Medical College, Gandhi Nagar, Kottayam, Kerala, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Manjunatha Sathyanarayanaprasad, Senior Resident, Department of Urology, Government Medical College, Gandhi Nagar, Kottayam-686008, Kerala, India.
E-mail: manju.bmc@gmail.com
Carcinoma developing at the Ureterosigmoidostomy (US) anastomosis is a complication known to us. Patients generally present late because of non specific symptoms like loin pain, loss of appetite and weight loss. Colonoscopy and abdominal imaging studies are recommended for early detection and prompt management of these malignancies. We report a case of mucin secreting adenocarcinoma of the colon, 26 years after US.
Colonoscopy, Complications, Extrophy
Case Report
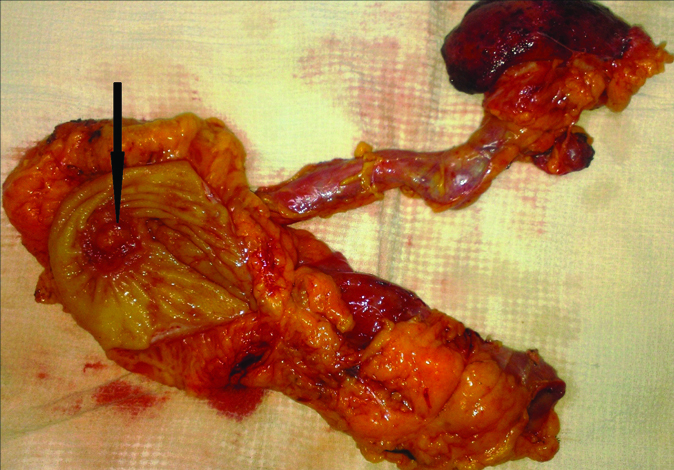
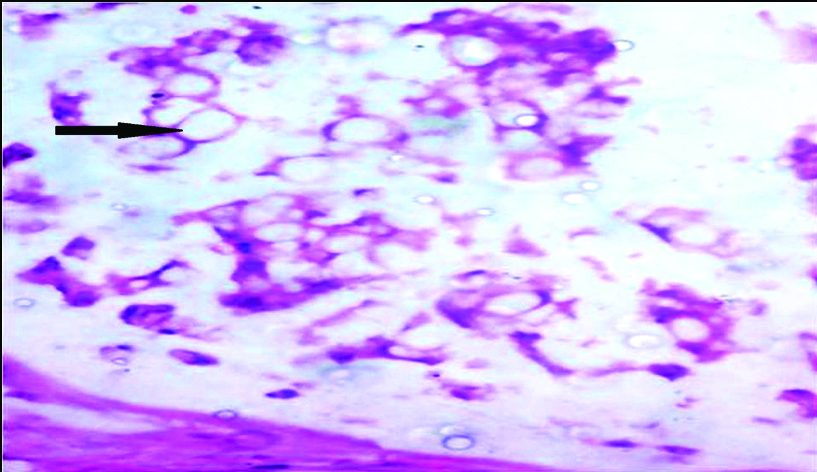
A 28-year-old male presented to Department of Urology with left flank pain since two months. He had undergone US along with cystectomy at the age of two years for ectopia vesicae. He was irregular at follow-up after that. Examination revealed a healthy male with no positive clinical findings other than external genitalia with epispadias. Ultrasonography of the abdomen showed right gross hydroureteronephrosis and left moderate hydroureteronephrosis. As serum creatinine was 3.4 mg/dL, bilateral percutaneous nephrostomies were done. Magnetic Resonance Imaging (MRI) showed bilateral gross hydroureteronephrosis. At colonoscopy, a nodular mass in the sigmoid colon at the US anastomotic site was seen, biopsy of which proved it to be adenocarcinoma. Technetium 99 diethylene triaminepentaacetic acid (99 mTc-DTPA) scan showed only 3% differential function in the right kidney. Sigmoid colectomy with right nephroureterectomy and left ureterostomy were performed [Table/Fig-1]. Postoperative period was uneventful. Histopathology of the resected sigmoid showed high grade mucinous carcinoma with signet ring cell variant invading through the muscularis propria and into serosa, resected margins were negative for tumour [Table/Fig-2]. Seven of the nine resected lymph nodes were positive for metastases. Patient received six cycles of 5-fluorouracil and oxaliplatin. At one year follow-up the patient remained asymptomatic and free of metastases.
Resected specimen of nephroureterectomy and sigmoidectomy with nodular tumour (arrow) at the ureterosigmoid anastomosis.
Photomicrograph showing signet ring cells (arrow) H&E staining (10X).
Discussion
The use of bowel segments in the urinary tract as part of the urinary diversion is associated with an increased risk of neoplasia. Most of these are adenomas and/or adenocarcinomas at the site of anastomosis of the ureter into the bowel. This complication is more common following US [1]. Gittes RF, used the term “urocolonic tumour” to describe this entity [2]. However, the exact pathogenesis of the development of present neoplasia is still unknown. The latent period for the occurrence of present neoplasia varies from 5 to 50 years. A significant majority of these patients are asymptomatic and hence, present with advanced disease [2].
The first ureterointestinal anastamosis was done by Simon about 150 years back and Hammer described the first colonic carcinoma developing at the site of ureteric implantation in 1929 [3]. The peak incidence of urocolonic tumour is usually in third or fourth decades of life, but it can also occur as early as seven years of age [3]. The neoplasia could be adenomas or adenocarcinomas and it is believed that malignant degeneration of the adenoma leads to adenocarcinoma [3]. Compared to the general population, the incidence of these tumours in patients with US is 100 to 550 times higher. The risk increases to a staggering 7000 fold if the diversion is performed before the age of 25 years. Adenomas and carcinomas developed after a mean latent period of 19.8 and 25.8 years respectively. The median age at diagnosis was 33 years [3].
Several theories have been proposed for the pathogenesis of development of malignancy after bowel interposition in the urinary tract. These include mixing of faecal and urinary materials; chronic inflammation, irritation and higher concentration of substances like nitrosamine, reactive oxygen species, epidermal growth factor, transforming growth factor, cyclo-oxygenase, mucin and ornithine transcarbamylase [4]. The most accepted theory is that of production of the carcinogen, nitrosamine, by the faecal flora. Occurrence of neoplasia in neobladders suggests that blending of urinary and faecal materials is not essential for the development of neoplasia [4].
Petterson L et al., in their case series of 25 patients, who underwent US for extrophy bladder in their childhood, described the occurrence of colorectal adenocarcinoma in seven patients. Of these seven, six were poorly differentiated and one was moderately differentiated. In this Swedish study, the mean time from US to the diagnosis of invasive adenocarcinoma was 38 years. Four patients in this study developed invasive malignancy even after complete resection of the ureteric orifice at re-diversion. According to the authors, the higher incidence of poorly differentiated malignancies could be due to the fluid nature of the faecal material (mixed with urine) instead of being solid in the colon. The fluid nature of the faecal matter may not produce obstructive symptoms and blood could be missed in the stools [5].
Conversion to an alternative form of diversion should be considered if polyps, dysplasia or neoplasia are found on follow-up. The previous anastomotic site in the sigmoid should be resected. Leaving behind the ureteric stumps may lead to development of neoplasia earlier as has been reported by Petterson L et al., and Strachan JR et al., [5,6]. However, there are reports of neoplasia occurring even after total excision of the anastomosis from the bowel [5].
The present patient was completely asymptomatic till he developed advanced disease. After the initial surgery 26 years back the patient did not follow up regularly. This report underscores the importance of strict follow-up regime for patients who have undergone or are undergoing US. Mainz II pouch is still being done in several centres. Patients should be well informed regarding the necessity and importance of lifelong colonoscopy.
In 2002, Woodhouse CRJ, proposed guidelines for monitoring patients with US. According to this, all patients should have a flexible sigmoidoscopy once a year starting 10 years after the initial surgery [3]. Those who have had conversion to another form of diversion later still need a yearly sigmoidoscopy unless it is known that the ureteric anastomoses were excised. Patients who have MAINZ II or Mansoura operations should have the flexible sigmoidoscopy to visualise the colon upto and just beyond the anastomosis. However, Petterson L et al., reported missing of two neoplasms which were at the distal ureteric end and not seen in the colon. Hence, they proposed that along with sigmoidoscopy, imaging studies of the abdomen should be done [3,5]. Fichtner J, and Austin M, and Kälble T, suggested that surveillance sigmoidoscopy be initiated at three and five years post surgery respectively [7,8].
Conclusion
Long term surveillance in the form of annual colonoscopy is recommendable after ureterosigmoidostomy.
[1]. Kälble T, Hofmann I, Thüroff JW, Stein R, Hautmann R, Riedmiller H, Secondary malignancies in urinary diversionsUrologe A 2012 51:502-06. [Google Scholar]
[2]. Gittes RF, Carcinogenesis in ureterosigmoidostomyUrol Clinics of North America 1986 13:201-05. [Google Scholar]
[3]. Woodhouse CRJ, Guidelines for monitoring of patients with ureterosigmoidostomyGut 2002 51(Suppl V):v15-16.10.1136/gut.51.suppl_5.v1512221034 [Google Scholar] [CrossRef] [PubMed]
[4]. Jian PY, Adenocarcinoma following urinary diversionCan Urol Assoc J 2012 6(2):e77-80.10.5489/cuaj.1106522511440 [Google Scholar] [CrossRef] [PubMed]
[5]. Pettersson L, Tranberg J, Abrahamsson K, Pettersson S, Sillen U, Jonsson O, Half century of followup after ureterosigmoidostomy performed in early childhoodJ Urol 2013 189(5):1870-756.10.1016/j.juro.2012.11.17923220244 [Google Scholar] [CrossRef] [PubMed]
[6]. Strachan JR, Rees HC, Cox R, Woodhouse CR, Mucin changes adjacent to carcinoma following ureterosigmoidosotmyEur Urol 1987 13(6):41910.1159/0004728392448143 [Google Scholar] [CrossRef] [PubMed]
[7]. Fichtner J, Follow-up after urinary diversionUrol Int 1999 63(1):40-45.10.1159/00003041710592489 [Google Scholar] [CrossRef] [PubMed]
[8]. Austen M, Kälble T, Secondary malignancies in different forms of urinary diversion using isolated gutJ Urol 2004 172(3):831-38.10.1097/01.ju.0000134890.07434.8e15310979 [Google Scholar] [CrossRef] [PubMed]