Cytokines TNF-Alpha and IL-8 Gene Polymorphisms in Sickle Cell Anaemia Patients under Hydroxyurea Treatment
Fathelrahman M Hassan1, Faisal M Alzahrani2
1 Associate Professor, Department of Clinical Laboratory Science, College of Applied Medical Science, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia.
2 Assistant Professor, Department of Clinical Laboratory Science, College of Applied Medical Science, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Fathelrahman M Hassan, P.O. Box: 1983, Dammam-31441, Saudi Arabia.
E-mail: fathmaga@yahoo.com
Introduction
Sickle Cell Anaemia (SCA) is an inherited disorder characterised by homozygosis for the mutation that causes Haemoglobin S (Hb S) production. SCA patients have increased serum levels of circulating Tumour Necrosis Factor Alpha (TNF-α) and Interleukin 8 (IL-8) during crisis events, occasions because of increased production from inflammatory cells, glia and neurons, these inflammatory molecules additionally contribute to the advanced mechanisms concerned in vascular occlusion events.
Aim
This study aimed to investigate polymorphisms in the TNF-alpha and IL-8 genes, their association with the haematological changes, to investigate the association between the TNF-alpha and IL-8 gene polymorphisms and the TNF-alpha and IL-8 serum levels in Saudi SCA patients presented with or without treatment in comparison to healthy individuals.
Materials and Methods
The study included 87 SCA patients diagnosed as homozygous for Haemoglobin S (Hb S; using haemoglobin electrophoresis methods and High-Performance Liquid Chromatography (HPLC) and attended at hereditary blood disease centre (Al-Ahsa; Saudi Arabia) for follow-up. The patients of both genders in all age groups were subdivided into two groups; 27 of the patients were undergoing hydroxyurea treatment (AHU) and 60 patients without hydroxyurea treatment against 30 healthy individual setting as control group. The collected data were analysed using the STATA SE 10 and GraphPad Prism 5.0.
Results
TNF-α and IL-8 levels were significantly higher within the plasma of SCA individuals compared to control individuals. The GG and AA genotypes of TNF-alpha-308G>A were associated with the increase in the serum levels of TNF-alpha in SCA patients. While, AA and TT genotypes -251A>T IL-8 gene polymorphism was associated with increase in the serum levels of IL-8 in SCA patients.
Conclusion
The haematological investigations of SCA further highlight the contribution of genetic modifications to the risk of clinical genotypes to understand the association of serum levels of TNF-α and IL-8 in patients under HU compared to their gene polymorphism.
Cytokine, Haemoglobin S, Vascular occlusion
Introduction
Sickle cell anaemia is an inherited disorder characterised by homozygosis for the mutation that causes Hb S production [1]. Genetic problems, together with Sickle Cell Disease (SCD), are very commonplace in Saudi Arabia [2,3]. Unfortunately, even though the progress of SCD has been decreasing in each location of Saudi Arabia, current efforts of Saudi to deal with this disorder was inadequate. The first pronounced case of SCD within the Eastern province turned into pronounced within the 1960s [2].
Although, the molecular abnormality resulting in the sickle sequence is the same to haplotypes, but there was a variation within the clinical manifestations and severity associated to SCD. The clinical constitution of SCD is claimed to be multigenic [4]. The sickle genotype, beta globin haplotype, and alternative genes, unlinked to the beta globin locus, participate within the relevant pathological events that cause modification of the phenotypic expression of the sickle gene [4]. Many other milder diseases such as; alpha thalassaemia were associated with increased Hb F [5] and these genetic defects were also found in SCD with high frequency in the Eastern Saudi Arabia [6,7].
TNF-alpha and IL-8 are pro-inflammatory molecules involved in endothelial cell and leukocyte activation, macrophage stimulation, affinity of leukocyte surface molecules, endothelial receptors and leukocyte chemotaxis and recruitment [8-11]. SCA patients have raised serum levels of circulating TNF-alpha and IL-8 and through crisis events [12,13]; these inflammatory molecules additionally contribute to the advanced mechanisms concerned in vascular occlusion events. Thus, aberrations in cell activation and interaction, the pro-inflammatory and oxidising agent profiles, genetic background and environmental factors presumably end in repeated vascular events [14,15].
Changes within the cytokine balance in SCA patients are a crucial risk issue for the prevalence of clinical events [16]. Moreover, inter-patient variations in cytokine levels can be attributed to gene polymorphisms, notably the A alleles of -308 G>A and -251 A>T, which are positioned in the promoter regions of the TNF and IL-8 genes, respectively, and have been associated with higher TNF-alpha and IL-8 transcript levels [17,18]. The present study investigated polymorphisms in the TNF-alpha and IL-8 genes and their association with their serum levels and haematological changes in Saudi SCA patients presented with hydroxyurea treatment or without treatment in comparison to healthy individuals.
Materials and Methods
A cross-sectional study including 87 (60 Sickle cell anaemia, SCA and 27 SCA under hydroxyurea therapy, AHU) patients selected from the haematology outpatient clinic of the Hereditary Blood Disease Centre (Al-Ahsa; Saudi Arabia) during the period from May 2014 to April 2016. Clinical data were collected from the patients’ medical records, and demographic data were obtained by interviews with patients or their guardians/parents. All patients were not in crisis and had not received blood transfusions during the previous three months. None of the patients was taking anti-inflammatory drug medication at the time of the study, while patients under hydroxyurea treatment had been taking the dose of 20-30 mg/kg/day for a minimum of three months. The control group included 30 individuals who attended the College of Medicine, King Faisal University (Al-Ahsa), and these individuals were in different ages- and both sexes as patients group. The control individuals had normal haemoglobin profiles and lacked a history of anaemia, inflammatory conditions and haematological diseases. Composed and verbal consent was obtained from all patients and controls within the study in accordance with the Declaration of Helsinki of 1975, as revised in 2000 and approved by Ethical Committee of the college.
Blood samples were collected from both patients and control under aseptic conditions. Haematological analyses were performed using an electronic cell counter (Coulter Counter T890, Brea, USA). The haemoglobin profile was analysed by HPLC (Bio-Rad, USA). Beta S-globin gene haplotypes was isolated from blood leukocytes using the GFXTM Genomic Blood DNA Purification Kit (AMER sham, USA). The TNF-alpha-308G>A and IL-8-251A>T gene polymorphisms were investigated with PCR and Restriction Fragment Length Polymorphism (RFLP) techniques as previously represented [19-22]. The sequences of the primers used were: TNF alpha forward: 5’GCG ACG TGG AAC TGG CAG AAG3’; TNF alpha reverse: 5’GGT ACA ACCCATCGG CTG GCA3’ and IL-8 forward: 5′-CCACCCCAAATTTATCAAAGAA-3′; IL-8 reverse: 5′-CAGACAGAGCTCTCTTCCATCA-3′. Serum cytokine levels of TNF-alpha and IL-8 l were measured with an Enzyme- Linked Immunosorbent Assay (ELISA) (BD Biosciences, USA), according to the manufacturer’s instructions, with cut-off levels of 67.8 pg/mL and 615.0 pg/mL for TNF-alpha and IL-8, respectively.
Statistical Analysis
The collected data analysed used Statistical Data Analysis (STATA) SE 10 and GraphPad Prism 5.0. A p-value of less than 0.05 was considered statistically significant. The baseline characteristics are presented as the means and proportions of the selected variables. Parametric ANOVA analyses were used to compare the means among two or more groups of interval variables that were normally distributed and not normally distributed, respectively. Fisher’s-exact test and the expected frequency in the tables were considered.
Results
This study included a total of 87 SCA patients with or without hydroxyurea treatment subdivided into two groups; 60 SCA patients (31 male and 29 females; 33.1±1.3 years) and 27 AHU patients (13 male and 14 females; 33.7±1.3 years) against 30 healthy individuals as control group. The independent haematological analysis shown statistically significant variation between the groups and a significant difference between the mean serum TNF-alpha level (p=0.0001) and the mean serum IL-8 levels (p=0.0001) in SCA patients compared to the AHU groups as described in [Table/Fig-1].
Haematological changes and cytokines of SCA patients and patients under hydroxyurea treatment (AHU) compared to healthy controls.
| Laboratory analysis | Mean±SD |
|---|
| Controls (n=30) | SCA (n=60) | AHU (n=27) |
|---|
| Male/Female | 21/9 | 31/29 | 13/14 |
| Age (years) | 36.6±1.2 | 33.1±1.3 | 33.7±1.3 |
| RBC (×1012/L) | 3.9±1.0 | 2.5±1.6 (0.0001)* | 3.0±1.5 (0.0003) |
| Haematocrit (%) | 43.4±2.6 | 22.3±1.9 (0.0001)* | 26.1±1.8 (0.0001)* |
| Hb (gm/dL) | 12.2±1.5 | 6.8±1.5 (0.0001)* | 9.3±2.1 (0.0001)* |
| Mean corpuscular volume (fL) | 90.2±2.3 | 92.2±1.8 (0.0001)* | 111.6±2.2 (0.0001)* |
| Mean corpuscular Hb (μL) | 31.1±1.2 | 30.5±1.2 (0.0279) | 33.0±2.0 (0.0001)* |
| WBC (×109/L) | 6.3±1.1 | 10.2±1.8 (0.0001) | 7.2±1.9 (0.0309) |
| Hb F (%) | 3.3±1.4 | 6.5±1.7 (0.0001) | 11.6±1.8 (0.0001)* |
| TNF-α (pg/mL) | 10.2±5.1 | 46±1.2 (0.0001) * | 26.8±5.8 (0.0001) * |
| IL-8 (pg/mL) | 11.9±3.2 | 39±1.8 (0.0001) * | 24±1.7 (0.0001) * |
*p<0.05 is significantly different among SCA and AHU patients compared with controls
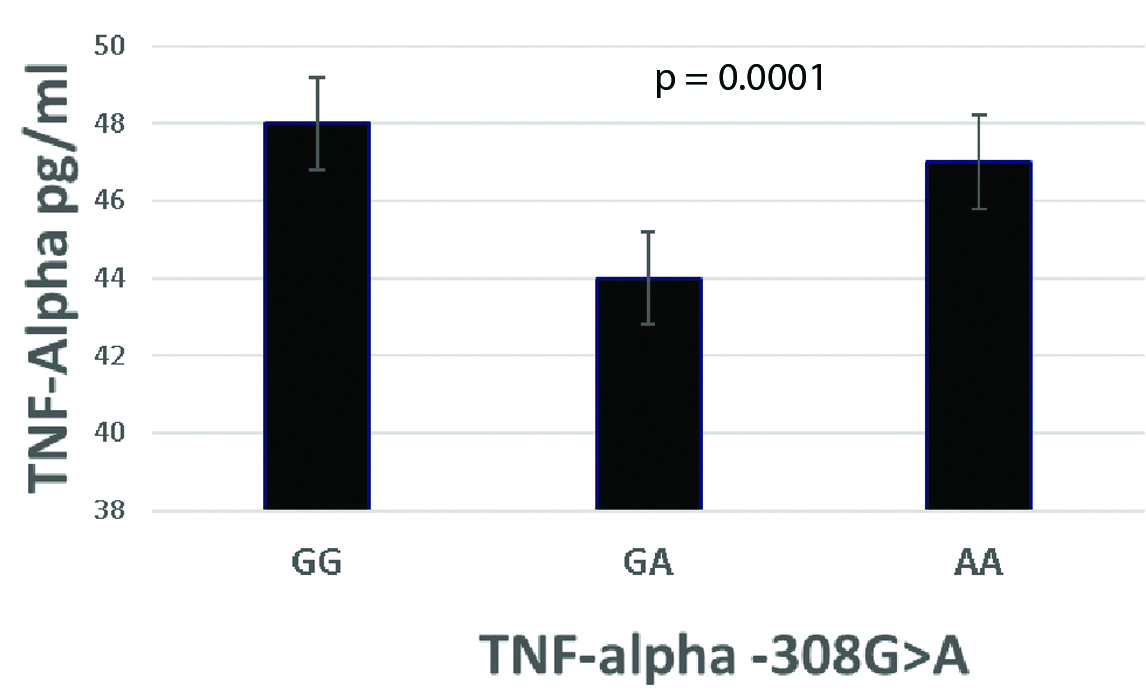
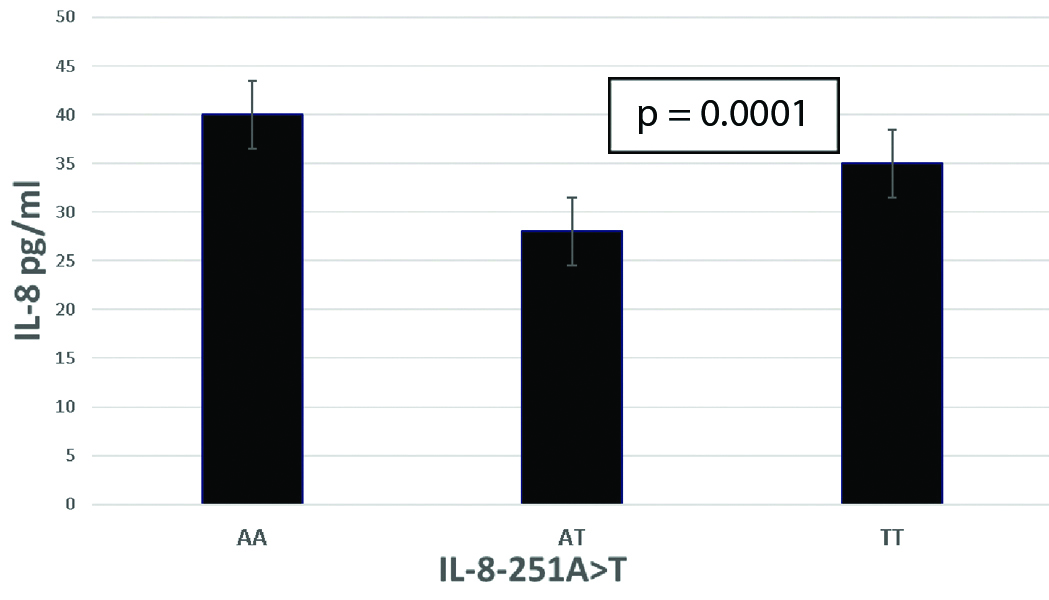
[Table/Fig-2] shows the GG and AA genotypes of the TNF-alpha -308G>A gene polymorphism was associated with the highest serum levels of TNF compared to GA genotypes (p=0.0001), while the AA and TT genotypes of the IL-8 -251A>T gene polymorphism were statistically significantly associated with highest serum levels of IL-8 compared to AT genotype (p=0.0001) as shown in [Table/Fig-3].
The mean of TNF-α serum levels compared to TNF-alpha -308G > A gene polymorphism in SCA patients.
The mean of IL-8 serum levels compared to IL-8-251A>T gene polymorphism in SCA patients.
The genotype frequencies were in Hardy-Weinberg equilibrium, the frequencies of IL-8-251A>T and TNF-alpha-308G>A gene polymorphisms of the 60 SCA patient, 27 AHU patients and 30 healthy control group were analysed, as evident in [Table/Fig-4].
The frequencies of IL-8 -251A>T and TNF-alpha -308G>A gene polymorphisms in SCA and hydroxyurea treatment patents (AHU) compared to healthy controls in Hardy-Weinberg equilibrium.
| Genotype | Genotype frequency N (%) |
|---|
| Control (n=30) | SCA (n=60) | AHU (n=27) |
|---|
| IL-8*-251A>T | AA | 4 (13.3) | 9 (15.0) | 3 (11.1) |
| AT | 15 (50.0) | 29 (48.3) | 14 (51.9) |
| TT | 22 (36.7) | 22 (36.7) | 10 (37.0) |
| p*=0.9753 | p*=0.9656 |
| TNF-α-308G>A | GG | 21 (70.0) | 37 (61.7) | 19 (70.4) |
| GA | 6 (20.0) | 21 (35.0) | 6 (22.2) |
| AA | 3 (10.0) | 2 (3.3) | 2 (7.4) |
| p*=0.1921 | p*=0.9324 |
*Fisher-test for p<0.05 was recorded in IL-8 of SCA and AHU patients compared with controls
Discussion
TNF-alpha, that is made by macrophages and T cells. It is a strong cytokine with a large range of pro-inflammatory activities as well as the activation of epithelial tissue [23]. These characteristics of TNF-alpha and its serum levels were related to the risk of SCA. Lanaro C et al., discovered an association between circulating levels and a rise in mRNA expression of TNF-alpha which confirmed the characteristic of a pro-inflammatory state in SCA patients [12]. Moreover, Pathare A et al., determined a rise of TNF-alpha concentration during SCA crisis [24]. In the present study, higher levels of TNF-alpha were detected in SCA patients compared with the control group. This result was in agreement to the previous researches. A similar study done by Wilson AG et al., recommended that A gene of TNF-alpha 308 GA was closely associated with transcriptional activity defect, raised its expression, which in turn relates to TNF-alpha levels [30].
Cytokine IL-8 levels were considerably raised in SCA patients compared to the control group (p-value) in which the polymorphism frequency and serum levels were increased due to its transcriptional activity, these aspects in agreement with previous studies of A allele in IL-8 [17,18,31] which confirmed A alleles of -251A>T related to higher IL-8 transcript levels with clinical importance in SCA patients. In the present study; Hb S was correlated to IL-8 which was confirmed by the haematological changes in SCA patients and patients under hydroxyurea treatment (AHU). SCD crisis, which was supported by a previous report could be determined by increased IL-8 levels because of the different pro-inflammatory markers related to SCA in patients throughout vascular occlusion episodes [13]. Elevated serum level of IL-8 was a marker of poor prognosis among AHU patients. Hb F, Hb level and haematocrit decrease in intravascular haemolysis, because of the SCA alternation crisis [29,32].
Conclusion
Although, this study was carefully done, there were still limitations; the sample size was small and lack of information constitutes a shortcoming of the study. Therefore, future studies with large sample size are needed in different ethnic groups with carefully matched cases and controls to confirm the results of our TNF and the -251A>T IL-8 polymorphism and other gene polymorphisms as a disease susceptibility factor in SCD.
Conclusion
The results presented here indicate the importance of the genotypes of the TNF and the -251A>T IL-8 polymorphism genes in the hydroxyurea therapy and patients follow-up during crisis of SCA and considering the important roles of these cytokines in SCA pathophysiology, further investigations are necessary to identify factors that are responsible for increased serum TNF-alpha and IL-8. This polymorphism when investigated with other genetic factors may unravel the pathogenesis of elevated pro-inflammatory cytokines in SCA. The present study emphasises that the identification of new genetic biomarkers and their association with classical markers is important to identify the different SCA phenotypes and their effects on patient outcome.
*p<0.05 is significantly different among SCA and AHU patients compared with controls
*Fisher-test for p<0.05 was recorded in IL-8 of SCA and AHU patients compared with controls
[1]. Steinberg MH, Rodgers GP, Pathophysiology of sickle cell disease: Role of cellular and genetic modifiersSeminars in Hematology 2001 38:299-306.10.1016/S0037-1963(01)90023-X [Google Scholar] [CrossRef]
[2]. Kulozik AE, Wainscoat JS, Serjeant GR, Kar BC, Al-Awamy B, Essan GJ, Geographical survey of b-globin gene haplotypes: Evidence for an independent Asian origin of the sickle cell mutationAm J Hum Genet 1986 39:239-44. [Google Scholar]
[3]. Nagel RL, Fleming AF, Genetic epidemiology of the beta s geneBaillieres Clin Haematol 1992 5:331-65.10.1016/S0950-3536(11)80023-5 [Google Scholar] [CrossRef]
[4]. El-Hazmi MA, Warsy AS, Appraisal of sickle-cell and thalassaemia genes in Saudi ArabiaEast Mediterr Health J 1999 5:1147-53. [Google Scholar]
[5]. El-Hazmi MA, Clinical and haematological diversity of sickle cell disease in Saudi childrenJ Trop Pediatr 1992 38:106-12.10.1093/tropej/38.3.1061380566 [Google Scholar] [CrossRef] [PubMed]
[6]. Stuart MJ, Nagel RL, Sickle-cell diseaseLancet 2004 364:1343-60.10.1016/S0140-6736(04)17192-4 [Google Scholar] [CrossRef]
[7]. Chui DH, Dover GJ, Sickle cell disease: No longer a single gene disorderCurr Opin Pediatr 2001 13:22-27.10.1097/00008480-200102000-0000411176239 [Google Scholar] [CrossRef] [PubMed]
[8]. Hebbel RP, Mohandas N, Cell adhesion and microrheology in sickle cell disease. In: Steinberg MH, Forget BG, Higgs DR, Nagel RL, editors. Disorders of hemoglobin: genetics, phatophysiology and clinical management 1999 New YorkCambridge University Press:527-49. [Google Scholar]
[9]. Assis A, Conran N, Canalli AA, Lorand-Metze I, Saad ST, Costa FF, Effect of cytokines and chemokines on sickle neutrophil adhesion to fibronectinActa Haematol 2005 113:130-36.10.1159/00008345115802892 [Google Scholar] [CrossRef] [PubMed]
[10]. Abbas AK, Lichtman AH, Pillai S, Cellular and molecular immunology 2007 Sixth EditionPhiladelphiaSaunders Elsevier [Google Scholar]
[11]. Hull J, Ackerman H, Isles K, Usen S, Pinder M, Thomson A, Unusual haplotypic structure of IL8, a susceptibility locus for a common respiratory virusAm J Hum Genet 2001 69:413-19.10.1086/32129111431705 [Google Scholar] [CrossRef] [PubMed]
[12]. Lanaro C, Franco-Penteado CF, Albuqueque DM, Saad ST, Conran N, Costa FF, Altered levels of cytokines and inflammatory mediators in plasma and leukocytes of sickle cell anaemia patients and effects of hydroxyurea therapyJ Leukoc Biol 2009 85:235-42.10.1189/jlb.070844519004988 [Google Scholar] [CrossRef] [PubMed]
[13]. Goncalves MS, Queiroz IL, Cardoso SA, Zanetti A, Strapazoni AC, Adorno E, Interleukin 8 as a vaso-occlusive marker in Brazilian patients with sickle cell diseaseBraz J Med Biol Res 2001 34:1309-13.10.1590/S0100-879X200100100001111593306 [Google Scholar] [CrossRef] [PubMed]
[14]. Hebbel RP, Adhesive interactions of sickle erythrocytes with endotheliumJ Clin Invest 1997 100:S83-86. [Google Scholar]
[15]. Mantovani A, Sozzani S, Vecchi A, Introna M, Allavena P, Cytokine activation of endothelial cells: new molecules for an old paradigmThromb Haemost 1997 78:406-14.10.1055/s-0038-16575619198188 [Google Scholar] [CrossRef] [PubMed]
[16]. Booth C, Stephen B, Obaro K, Infection in sickle cell disease: A reviewInter J Infect Dis 2010 14:e2-e12.10.1016/j.ijid.2009.03.01019497774 [Google Scholar] [CrossRef] [PubMed]
[17]. Ruo-long Z, Hua Z, Wen-long J, Tumour necrosis factor-alpha 308G>A polymorphism and risk of rheumatic heart disease: a meta-analysisScientific Reports 2014 4:473110.1038/srep0473124751687 [Google Scholar] [CrossRef] [PubMed]
[18]. Hacking D, Knight JC, Rockett K, Brown H, Frampton J, Kwiatkowski DP, Increased in vivo transcription of an IL-8 haplotype associated with respiratory syncytial virus disease-susceptibilityGenes Immun 2004 5:274-82.10.1038/sj.gene.636406715085176 [Google Scholar] [CrossRef] [PubMed]
[19]. Sutton M, Bouhassira EE, Nagel RL, Polymerase chain reaction amplification applied to the determination of beta-like globin gene cluster haplotypesAm J Hematol 1989 32:66-69.10.1002/ajh.28303201132757004 [Google Scholar] [CrossRef] [PubMed]
[20]. Dode C, Krishnamoorthy R, Lamb J, Rochette J, Rapid analysis of -alpha 3.7 thalassaemia and alpha alphaalpha anti 3.7 triplication by enzymatic amplification analysisBr J Haematol 1993 83:105-11.10.1111/j.1365-2141.1993.tb04639.x8435317 [Google Scholar] [CrossRef] [PubMed]
[21]. Seitzer U, Swider C, Stuber F, Suchnicki K, Lange A, Richter E, Tumour necrosis factor alpha promoter gene polymorphism in sarcoidosisCytokine 1997 :787-90.10.1006/cyto.1997.02249344512 [Google Scholar] [CrossRef] [PubMed]
[22]. Heinzmann A, Ahlert I, Kurz T, Berner R, Deichmann KA, Association study suggests opposite effects of polymorphisms within IL8 on bronchial asthma and respiratory syncytial virus bronchiolitisJ Allergy Clin Immunol 2004 114:671-76.10.1016/j.jaci.2004.06.03815356575 [Google Scholar] [CrossRef] [PubMed]
[23]. Pathare A, Al Kindi S, Alnaqdy AA, Daar S, Knox-Macaulay H, Dennison D, Cytokine profile of sickle cell disease in OmanAm J Hematol 2004 77(4):323-28.10.1002/ajh.2019615551290 [Google Scholar] [CrossRef] [PubMed]
[24]. Pathare A, Kindi SA, Daar S, Dennison D, Cytokines in sickle cell diseaseHematology 2003 8:329-37.10.1080/1024533031000160471914530175 [Google Scholar] [CrossRef] [PubMed]
[25]. Hibbert JM, Hsu LL, Bhathena SJ, Irune I, Sarfo B, Creary MS, Proinflammatory cytokines and the hypermetabolism of children with sickle cell diseaseExp Biol Med (Maywood) 2005 230(1):68-74.10.1177/15353702052300010915618128 [Google Scholar] [CrossRef] [PubMed]
[26]. Bijan K, Ali Reza M, Reza N, Mastaneh A, Somayeh G, Fariba S, Altered levels of pro-inflammatory cytokines in sickle cell disease patients during vaso-occlusive crises and the steady state conditionEur Cytokine Netw 2013 24:25-52.10.1684/ecn.2013.032823608554 [Google Scholar] [CrossRef] [PubMed]
[27]. Etienne-Julan M, Belloy MS, Decastel M, Dougaparsad S, Ravion S, Hardy-Dessources MD, Childhood sickle cell crises: clinical severity, inflammatory markers and the role of interleukin-8Haematologica 2004 89(7):863-64. [Google Scholar]
[28]. Banerjee N, Nandy S, Kearns JK, Bandyopadhyay AK, Das JK, Majumder P, Polymorphisms in the TNF-{alpha} and IL10 gene promoters and risk of arsenic-induced skin lesions and other nondermatological health effectsToxicol Sci 2011 121:132-39.10.1093/toxsci/kfr04621357384 [Google Scholar] [CrossRef] [PubMed]
[29]. Cajadoa C, Cerqueira B, Coutoa F, Moura-Netoa J, Vilas-Boasa W, Dorea M, TNF-alpha and IL-8: Serum levels and gene polymorphisms (–308G>A and –251A>T) are associated with classical biomarkers and medical history in children with sickle cell anaemiaCytokine 2011 56(2):312-17.10.1016/j.cyto.2011.07.00221802960 [Google Scholar] [CrossRef] [PubMed]
[30]. Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW, Effects of a polymorphism in the human tumour necrosis factor alpha promoter on transcriptional activationProc Natl Acad Sci USA 1997 94:3195-99.10.1073/pnas.94.7.31959096369 [Google Scholar] [CrossRef] [PubMed]
[31]. Beena P, Marcus K, Jessica H, Johannes F, Andrea H, Impact of IL8 and IL8- Receptor alpha polymorphisms on the genetics of bronchial asthma and severe RSV infectionsClin Mol Allergy 2006 4:2 [Google Scholar]
[32]. Kato GJ, Gladwin MT, Steinberg MH, Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypesBlood Rev 2007 21(1):37-47.10.1016/j.blre.2006.07.00117084951 [Google Scholar] [CrossRef] [PubMed]