Gliomas are the most prevalent primary intracranial tumour, representing 81% of malignant brain tumours [1]. In Egypt, CNS neoplasms represent about 3% of primary malignant tumours. They are the most prevalent solid tumours in children. The gliomas represent approximately one-third of primary central nervous system tumours [2].
Astrocytic gliomas are heterogeneous group of malignancies. They are categorised into low grade astrocytomas (Grade I-II), anaplastic astrocytomas (Grade III) and glioblastoma (Grade IV) [3]. The therapeutic resistance nature and high invasiveness, of glioma may be attributable to the lack of reliable tumour markers for molecular targets against the prognosis. Therefore, detection of prognostic markers could help to denote more clearly, the use of adjuvant therapy, and to assess the accurate prognosis [4,5].
EMMPRIN, also known as CD147, is an adhesion molecule, a member of the immunoglobulin family and a Type I transmembrane glycoprotein [6,7].
EMMPRIN levels significantly correlated with WHO grades in human meningiomas and astrocytomas [8]. Higher EMMPRIN expression observed in gliomas when compared to nearby brain tissue, EMMPRIN is noted to be a strong prognostic marker. Its expression intensely correlated with the late stages of the disease [9]. Strongly positive and correlative EMMPRIN and Matrix Metalloproteinase-2 (MMP-2) expression was noted in high versus low-grade glioma and normal brain, and EMMPRIN+/ MMP2+ expression was considered to be prognostic factors for low survival rates [10].
Some of the prognostic markers for astrocytoma are CD24, CD63, CD99, CD151, CD155, Ezrin, Fascin-1, MMP-2, MMP-9, MMP-13, Cathepsin B and Cyclin D1 [11].
The important function of EMMPRIN in tumour progression by various studies denoted that EMMPRIN correlated with clinical prognosis of some malignancies such as breast cancer, salivary duct carcinoma, prostate cancer, pulmonary adenocarcinoma, colorectal cancer and bladder cancer [12-17].
Thus, the aim of this study was to evaluate the immunohistochemical expression of EMMPRIN marker in cases of CNS astrocytomic tumours and correlation between EMMPRIN immunohistochemical expression with clinical and pathological features of such tumours, such as site, gender, age and the WHO tumour grade.
Materials and Methods
This is a cross-sectional retrospective study which included 60 cases of astrocytic CNS tumours obtained through collection of archived paraffin blocks of astrocytic tumour of CNS, from the Department of Pathology, Faculty of Medicine, Cairo University, Cairo, Egypt, during the period January 2016 till December 2016. Inclusion criteria included cases diagnosed as astrocytic tumours and adequate biopsy. However, the exclusion criteria included tiny biopsies, biopsies with extensive haemorrhage and necrosis. The medical records were revised which included clinical and histopathological data such as age, gender, site and the grade of astrocytic tumours. The study was conducted after the approval of Ethical Pathology Committee in Faculty of Medicine, Cairo University (N-56-2016).
Each paraffin block was re-cut by rotator microtome at 5 μ thickness then mounted on glass slides to be stained by Haematoxlyin and Eosin (H&E) for histopathological re-evaluation. Histopathologic examination of H&E stained slides was performed under low power then high power for confirming the diagnosis. Histological grading for astrocytic tumours were classified according to WHO classification 2016 [18].
Immunohistochemistry
Paraffin blocks were cut and mounted on charged slides and stained manually for immunohistochemistry. The sections were deparaffinized in xylene, for 10 minutes, then dehydrated in descending series of ethanol (100%, 96%, 70%), followed by washes in TBS (0.05 mmol/L Tris-buffer physiological saline, pH 7.4-7.6), for 5 minute.
Antigen retrieval was achieved by heating the samples without boiling in 10 mmol/L sodium citrate buffer, pH 6.0 (200 mL) in a microwave oven. This treatment was conducted twice for 7 minutes. The sections were washed in TBS buffer for 30 minutes.
The endogenous peroxide was blocked by 0.3% hydrogen peroxide in methanol for 5 minutes. The sections were washed in TBS for 15 minute. To inhibit non-specific background staining; the samples were incubated in super block for 5-10 minutes at room temperature.
The primary antibody was monoclonal rabbit EMMPRIN antibody, purchased from Midco Trade Company, LOT NO.L16040766, at a dilution of 1:200 from the stock. The dilution was based on dilution experiments. The antibody was diluted with 20 mmol/L TBS, pH 7.4 (10 mmol/L CaCl2, 0.1% NaN3 and 1% BSA). The sections were incubated in the diluted antibody. The incubation took place in incubation boxes overnight.
The secondary antibody (4.5 μL biotinylated anti-mouse antibody in 1 mL of 1% BSA) was pipetted onto the sections and incubated in the moist box for 30 minutes. The secondary antibody was washed in TBS buffer for 15 minutes. The final staining was done in Diaminobenzidine Tetrahydrochloride (DAB) solution (49 mL TBS-buffer, 34 mg imidazole, 17 μL 30% hydrogen peroxide and 1 mL 30% DAB), for 5-15 minutes. The slides were washed with distilled water, 70% ethanol for 1 minute, then in distilled water. The nuclei were stained with Mayer’s haematoxylin for 30 seconds as counter stain. Extra stain was washed with tap water. The slides were then transferred through ascending ethanol series, and xylene before mounting.
Evaluation of Expression of EMMPRIN
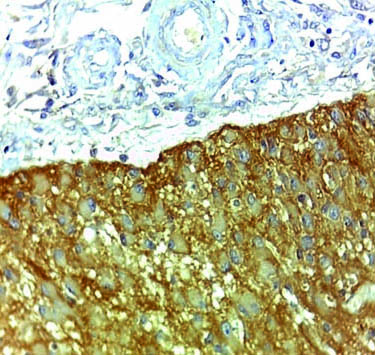
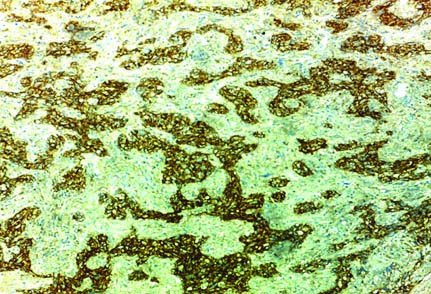
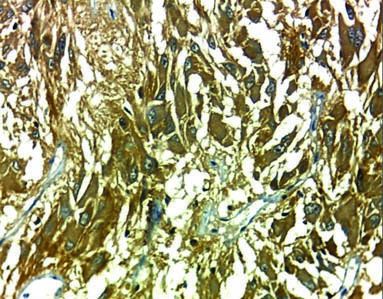
Tumour tissue sections were examined and scored under LEICA ICC50HD microscope at low power then high power magnification by four pathologists. Cytoplasmic and membranous staining for EMMPRIN was accepted as positive. The control was normal brain tissue as internal control and skin biopsy as external control as shown in [Table/Fig-1]. Each slide was evaluated according to staining extent and intensity. Staining intensity was also categorised into three groups, where 1=weak, 2=moderate and 3=strong [19]. The extent of staining was scored semi quantitatively and was calculated as the percentage of stained cells per 10 up to 50 high power field according to the size of tumour tissue, using (0 to 4) scale for expression, where 0=no expression, 1=1-25%, 2=26-50%, 3=51-75% and 4=76-100% [19]. Staining extent and intensity scores were added to give combined scores, which were categorised into four groups in which the score: 0-1=negative staining; 2-3=weak staining as shown in [Table/Fig-2]; 4-5=moderate staining; and 6-7=strong staining as shown in [Table/Fig-3,4,5,6 and Table/Fig-7]. For statistical purposes, EMMPRIN staining in tumour cells was further divided into; low expression [Score 2-3 and 4-5] and high expression [Score 6-7]. The results of EMMPRIN immunostaining in tumour cells were correlated with multiple prognostic factors such as age, sex, site of the tumour, and histopathologic WHO grade [19].
Skin as external control EMMPRIN immunohistochemical staining (magnification 10X).
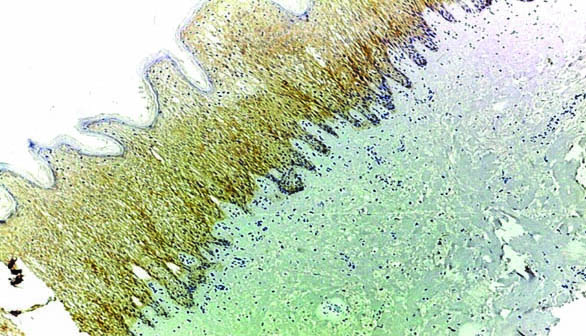
Case of pilocytic astrocytoma showing weak membranous and cytoplasmic staining of EMMPRIN (magnification 10X).
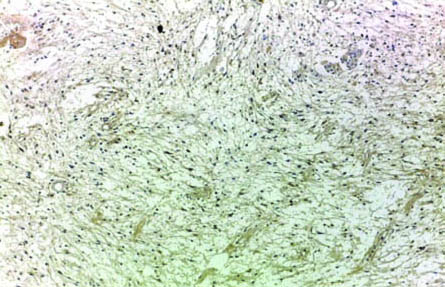
Case of anaplastic astrocytomas NOS showing strong membranous and cytoplasmic staining of EMMPRIN (magnification 40X).
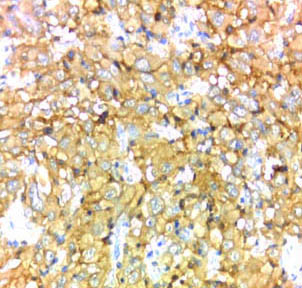
Case of glioblastoma NOS showing strong membranous and cytoplasmic staining of EMMPRIN (magnification 10X).
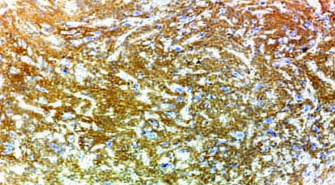
Case of glioblastoma NOS showing strong membranous and cytoplasmic staining of EMMPRIN (magnification 40X).
Case of glioblastoma NOS showing strong membranous and cytoplasmic staining of EMMPRIN (magnification 10X).
Case of subependymal giant cell astrocytoma strong membranous and cytoplasmic staining of EMMPRIN (magnification 40X).
Statistical Analysis
Microsoft excel 2010 was used for data entry and the Statistical Package for Social Science (SPSS version 21.0) was used for data analysis. Simple descriptive statistics (arithmetic mean and standard deviation) used for summary of quantitative data and frequencies used for qualitative data. Bivariate relationship was displayed in cross tabulations and comparison of proportions was performed using the chi-square test. Independent t-test was used to compare normally distributed quantitative data. All p-values were two-sided. The p-values <0.05 were considered significant.
Results
Clinico-pathological characteristics of the studied cases of CNS astrocytic tumours are shown in [Table/Fig-8].
Clinico-pathological characteristics of the studied cases of CNS astrocytic tumours and its correlation with EMMPRIN expression.
| Parameter | Number (%) | Low EMM PRIN expression | High EMM PRIN expression | p-value |
|---|
| Age |
| <40 years | 33 (55%) | 25 (58.1%) | 8 (47.1%) | 0.437 |
| >40 years | 27 (45%) | 18 (41.9%) | 9 (52.9%) |
| Gender |
| Male | 35 (58.3%) | 22 (51.2%) | 13 (76.5%) | 0.073 |
| Female | 25 (41.7%) | 21 (48.8%) | 4 (23.5%) |
| Tumour site |
| Spinal | 1 (1.7%) | 1 (2.3%) | 0 (0.0%) | 0.561 |
| Subtentorial | 11 (18.3%) | 9 (20.9%) | 2 (11.8%) |
| Supratentoral | 48 (80.0%) | 33 (76.7%) | 15 (88.2%) |
| Tumour grade |
| Grade 1 | 15 (25.0%) | 13 (30.2%) | 2 (11.8%) | 0.035 |
| Grade 2 | 13 (21.7%) | 12 (27.9%) | 1 (5.9%) |
| Grade 3 | 11 (18.3%) | 7 (16.3%) | 4 (23.5%) |
| Grade 4 | 21 (35.0%) | 11 (25.6%) | 10 (58.8%) |
Using the chi-square test and Independent t-test
As regard, EMMPRIN expression in the current study, 17 cases (28.3%) exhibited strong EMMPRIN expression, while 19 cases (31.7%) exhibited moderate expression and 24 cases (40%) exhibited weak expression. According to the scoring system used in the current study and previously described, the expression was further divided into two groups: weak and moderate expression which represented low expression and strong expression represented high expression. So, 43 cases (71.7%) showed low expression and 17 cases (28.3%) showed high expression as shown in [Table/Fig-9]. In the present study, 30.2% of Grade 1 astrocytomas showed low expression of EMMPRIN, 27.9% of Grade II astrocytomas showing low expression, 23.5% of Grade III astrocytomas showing high expression, 58.8% of Grade IV astrocytomas showing high expression of EMMPRIN. It suggested that EMMPRIN staining was significantly accounted for the elevation of invasion and metastasis ability of glioma with advanced grade.
The expression of EMMPRIN expression in the studied cases.
| EMMPRIN expression | Number of cases | Percentage |
|---|
| Low | 43 | 71.7% |
| High | 17 | 28.3% |
| Total | 60 | 100.0% |
The relationships between EMMPRIN expression and the clinico-pathological variables are shown in [Table/Fig-8]. There was significant correlation between the expression of EMMPRIN and WHO tumour grade (p-value=0.035).
Discussion
The age of the patients in the present study ranged from 2 to 74 years with mean age 37.23 years at the time of pathological diagnosis, 45% of patients were older than 40 while 55% of the patients were either 40 or younger than 40. Within this group 13.3% were in paediatric age group (2-10 years). Different results were obtained by Yang M et al., who reported that their patient’s age ranged from 14-78 years old at the time of pathological diagnosis with mean age 53.6 years. The variability of this data was due to difference in demographic features of populations [20].
In the current study, eight cases were in paediatric age group (2-10 years) at the time of pathological diagnosis. All were diagnosed as pilocytic astrocytomas. In a study by Gu J et al., 45 paediatric patients, aged (3 months to 12 years) were evaluated and revealed that 18 cases were diagnosed as low-grade astrocytomas (3 cases were pilocytic astrocytomas, 15 cases were diffuse astrocytomas) and 27 cases were high-grade astrocytomas (6 cases were anaplastic astrocytomas, 21 cases were diagnosed as glioblastoma) [10].
Male patients represented 58% of the studied cases, while 42% of patients were females, near findings were detected in the study done by Tian L et al., in which males represented 55% and females were 45% [9].
In the current study, there were no significant relationships between EMMPRIN expression and age, gender, and site of the tumour; this was in accordance with Tian L et al., and Yang M et al., who observed the same finding [9,20].
In the present study, 60 cases of astrocytic tumours were studied. According to the WHO classification Louis DN et al., cases were collected from paraffin embedded tissue sections sent to Department of Pathology, Kasr EL Ainy during the period from January 2016 till December 2016 [18]. A total of 21 cases (35%) were Grade IV glioblastoma, NOS as shown in [Table/Fig-10,11,12, and 13], 11 cases (18.3%) were Grade III anaplastic astrocytoma, NOS as shown in [Table/Fig-14], 13 cases (21.7%) were Grade II diffuse astrocytoma, NOS as shown in [Table/Fig-15], and 15 cases (25%) were Grade I (two cases of these were subependymal giant cell astrocytomas as shown in [Table/Fig-16]) and 13 cases were pilocytic astrocytomas. Glioblastoma NOS was considered to be the most frequent in the studied cases. While the study of Tian L et al., was performed on 306 patients, 19% of the cases were Grade 1, 20% of the cases were Grade 2, 25% of the cases were Grade 3 and 36% of the cases were Grade 4. However, the study of Yang M et al., was carried out on 206 cases of glioblastoma [9,20].
Case of gliosarcoma NOS (H&E; magnification 10X).
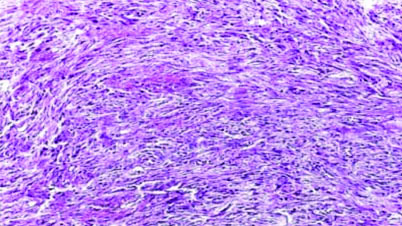
Case of glioblastoma NOS with excess bizarre giant cells (H&E; magnification 40X).
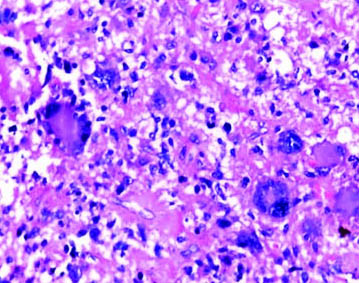
Case of glioblastoma NOS with microvascular proliferation (H&E; magnification 10X).
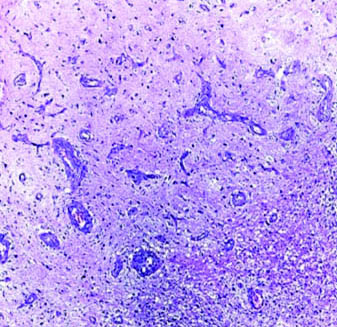
Case of glioblastoma NOS with palisading necrosis (H&E; magnification 10X).

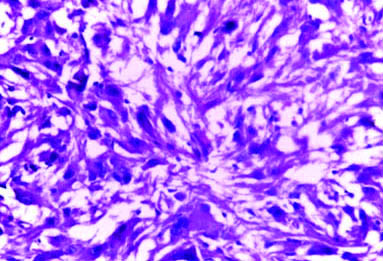
Case of anaplastic astrocytomas NOS (H&E; magnification 10X).
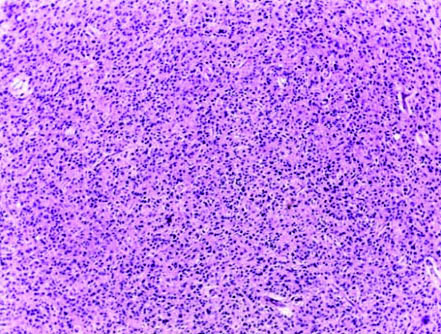
Case of diffuse astrocytomas NOS (H&E; magnification 10X).
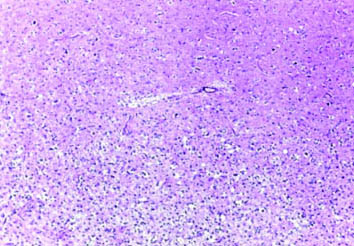
of subependymal giant cell astrocytoma (H&E; magnification 40X).
Considering the findings of the present study, there was significant correlation between the expression of EMMPRIN and WHO tumour grade (p-value=0.035). The level of expression of EMMPRIN was significantly higher in glioblastoma WHO Grade 4 than other grades of astrocytic tumours, which may explain the more aggressive nature of high grade astrocytic tumours. The study of Tian L et al., also showed significant relation of EMMPRIN with WHO grade of gliomas [9].
Riethdorf S et al., reported that glioblastoma (Grade 4 astrocytoma), considered as the most aggressive type of gliomas, showing strong EMMPRIN immunostaining than lower grades astrocytomas [21]. This coincided with the results of the current study.
Gu J et al., detected that strongly positive and directly correlative EMMPRIN and MMP-2 expression were detected in high-grade than low-grade gliomas and normal brain which is denoting prognostic significance of EMMPRIN and MMP-2. This was consistent with the finding of the present study [10].
Wightman SM reported that the protein basigin-2 (EMMPRIN) increases the expression of MMP enzymes, and its expression level is correlated with glioblastoma tumour grade, with basigin-2 (EMMPRIN) overexpression strongly correlated with tumour grade. One of its important function is the ability to initiate the expression and secretion of MMPs from nearby tissues, increasing the ability of the tumour to enlarge and metastasise [22].
Yin H et al., also detected that the expression of (EMMPRIN) was markedly associated with angiogenesis and WHO tumour grade; down regulation of (EMMPRIN) was leading to inhibition of glioma angiogenesis, proliferation and invasion. Yin H et al., study also used immunohistochemical technique which was performed on 101 glioma cases and 30 cases of normal brain obtained from brain injury to detect the expressions of EMMPRIN, CD34, and VEGF in tissue samples. EMMPRIN was positively expressed with the percentage of 0, 37.5, 44.8, 67.9, and 85.7 in normal tissues and WHO grades I-IV gliomas respectively, EMMPRIN was significantly expressed in the human gliomas (p<0.05) [23].
Limitation
The limitation of this study was the lack of molecular testing and therapeutic target for EMMPRIN in CNS astrocytic tumours due to high cost, the lack of reliable registry of the patient and follow-up, contributed to that the survival rate could not be assumed in the study.
Conclusion
A significant correlation was detected between the expression of EMMPRIN and WHO grading of CNS astrocytic tumour. Suggesting that, EMMPRIN should be used as prognostic marker in CNS astrocytoma. EMMPRIN is a novel and worthwhile therapeutic target for cancer, since it is implicated in many areas of tumour progression including invasion and migration. Future studies should be carried out on larger scale in correlation with other factors as survival rate and therapeutic effect to confirm the precise role of EMMPRIN in CNS astrocytic tumours.
Using the chi-square test and Independent t-test