Introduction
Electrolytes in the body including sodium (Na+), calcium (Ca2+), potassium (K+), chlorine (Cl-) and magnesium (Mg2+) play important physiological roles in the body such as enhancing enzyme activities, creating electrical gradients, promoting several metabolic and cellular activities, and ensuring normal homeostasis [1]. However, distortion or imbalance of the normal electrolyte level may lead to clinical abnormalities or disorders which are frequently associated with increased morbidity and mortality [2]. Electrolyte imbalance is frequently observed in clinical subjects and usually caused by several factors including gastrointestinal absorption capacity, nutritional deficiencies, acid-base abnormalities, pharmacological agents, renal disease, acute illness or diseases which can act alone or in combination [3-5].
Diabetes is one of the diseases which frequently lead to electrolyte distortion [4,6]. In a diabetic condition, high blood glucose increases plasma osmolarity which in turn creates an osmotic driving force that drifts water movement from the intracellular spaces to the extracellular spaces [7]. This osmotic drift and water movement has two major effects on electrolyte concentration in the body. It could lead to a dilutional effect lowering electrolyte concentration if they are extracellular, or increase the extracellular concentration if the water movement carries along intracellular electrolytes to the extracellular space especially in a state of insulin deficiency [8]. This osmotic drift leads to a condition termed as electrolyte disorder or imbalance. Both hyper- and hypo-electrolyte levels are observed in diabetes. Certain studies have shown hyperkalaemia, hypernatraemia, and hypermagnesaemia etc., to occur in diabetic patients as well as hypokalaemia and hyponatraemia are also possible due to osmotic diuresis, antidiabetic agents or exogenous insulin administration [9-12]. The derangement of chloride in diabetes remains unclear as very few studies have evaluated the extent of chlorine alteration among various populations [13].
Moreover, diabetic nephropathy, which is one of the complications of diabetes characterised by impaired renal function or failure can lead to electrolyte imbalance as elevated blood sugar damages the nephron, thereby altering electrolyte absorption and reabsorption [9].
Diabetes is a multifactorial disease which is associated with sex, age, blood pressure etc., [14]. Recently, the increasing prevalence of this disease is drifting from the high-income countries to middle and low-income countries, especially Nigeria which presents the greatest disease burden in Africa affecting over 1.2 million people [15]. However, scanty data is available on the electrolyte profile of diabetic patients in Nigeria especially in relation to the prevailing risk factors including renal dysfunction indices. Hence, this study was aimed to assess the serum electrolyte level of diabetic patients in order to evaluate the relationship of these electrolytes with renal dysfunction indices along other diabetes risk factors.
Materials and Methods
Study Population and Design: This was a continuation of an ongoing case—control study which recruited T2D patients and patients without diabetes (ND) of Nigerian ethnicity at Enugu State University Teaching Hospital (ESUTH) in Enugu Nigeria. The sample size (n) was calculated according to Charan and Biswas using the formula: n= 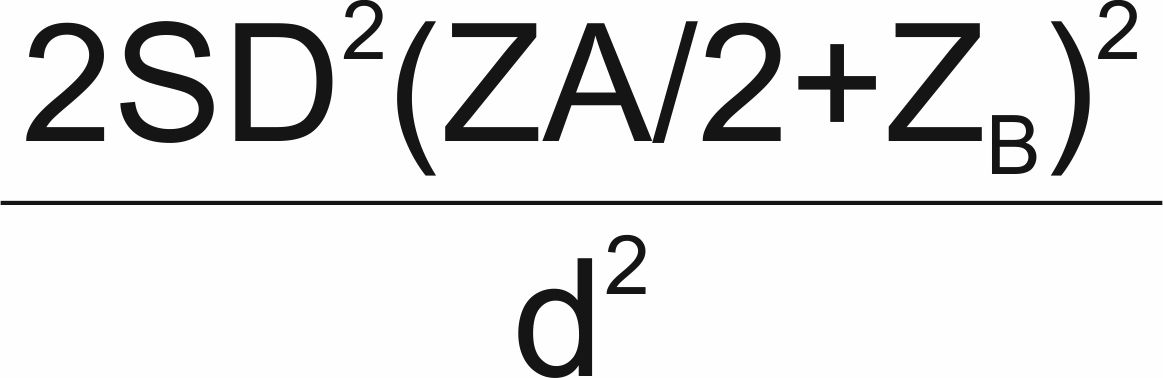 = 74.19. Where; SD = standard deviation, Za/2 = Zscore for type 1 error of 5%, ZB = Zscore at 95% power and d = Effect size from previous study [16,17]. Thus, approximately 74 participants were recruited for each arm (case and control).
= 74.19. Where; SD = standard deviation, Za/2 = Zscore for type 1 error of 5%, ZB = Zscore at 95% power and d = Effect size from previous study [16,17]. Thus, approximately 74 participants were recruited for each arm (case and control).
Ethics Approval and Consent to Participate: The study design was reviewed and approved by the Ethical Committee of ESUTH Enugu, Nigeria with approval number: ESUTHP/C-MAC/RA/034/174. The study was conducted in accordance to the guidelines of the Helsinki Declaration and written informed consent was obtained from all patients willing to participates in the study.
Inclusion/Exclusion Criteria: Outpatients above 30 years of age, who were not admitted at the hospital and without any critical or emergency health condition or complication, were recruited for the study. Pregnant and breastfeeding women, patients on diuretics as well as HIV positive patients were excluded from the study. Patients considered as T2D patients were diagnosed according the IDF criteria with hyperglycaemia and at least one-year history of the disease while non-diabetic control patients were those without T2D or hyperglycaemia [18].
Data Collection: Demographic information of patients, including age, sex and disease history was obtained by interview using a questionnaire. The resting blood pressures; SBP and DBP were measured using standardised procedures with an automatic sphygmomanometer. The height, weight and Waist Circumference (WC) of the patients were measured and the Body Mass index (BMI) calculated in Kg/m2.
Blood Collection and Laboratory Analysis: Blood (2ml) was collected from each patient and transferred into plain tubes without anticoagulants and fluoride tubes. The Fasting Blood Sugar (FBS) was measured from blood in fluoride tubes using AccuCheck glucometer. The blood in plain tubes was then centrifuged at 5000 rpm for 10 minutes to obtain serum. Serum electrolytes; K+, Na+ and Cl- were measured using kits (Teco Diagnostics, California USA). Urea was measured using a urea kit (Randox Laboratories Ltd., UK) and creatinine was measured using creatinine PAPSL kit (ELITech Clinical Systems SAS, France). All the measurements were done according to the manufacturer’s protocol. K+ was measured based on the method of Skeggs and Hochstrasser, Na+ was assayed based on the method of Maruna and Trinder with slight modifications [19-21]. Cl- was measured according to Schoenfeld and Lewellen [22]. Urea was assayed according to the method of Watt and Crisp and creatinine was measured based on the method of Owen A et al., [23,24].
Definition and Classification of Serum Electrolytes and Renal Function Indices
The following cut-off limits were taken for derangement of serum electrolytes and renal function indices according to the manufacturer’s guidelines. Serum Na+ level (mEq/L) was classified as hyponatraemia < 135, normal level was between 135 and 155 while > 155 as hypernatraemia. Serum K+ level (mEq/L) < 3.4 as hypokalaemia, normal level was between 3.4 and 5.3, and > 5.3 as hyperkalaemia. Serum Cl- level (mEq/L) was classified as hypochloraemia < 98, normal from 98 to 106 and >106 as hyperchloraemia. Urea level (mg/dL) was considered as hypoureaemia < 10, between 10 and 55 as normal, and > 55 as hyperureaemia. Creatinine level (mg/dL) was considered as low <0.55, between 0.55 and 1.15 as normal, from 1.16 to 3.99 as high and > 4 as very high (indicating renal damage).
Statistical Analysis
Data was analysed using Statistical Package for the Social Sciences (SPSS) version 16. Results were expressed as Mean±Standard Error of the Mean (SEM) and frequencies presented in Tables and Figures. Pearson chi-square (χ2) test was used to compare the proportional differences of electrolytes and renal function indices derangement in T2D and ND patients. Mean differences of electrolytes, creatinine, urea level and baseline parameters were compared between the T2D and ND patients using parametric independent sample t-test. Correlation between various study parameters (electrolytes and renal function indices) and risk factors (age, SBP, DBP and FBS) was done using Pearson correlation test. A statistically significant difference was considered at p-value less than 0.05.
Results
Baseline Characteristic of Participants: A total of 147 patients participated in the study of which 72 (49.0 %) were T2D patients while 75 (51.0%) were ND patients with an age range between 30 and 92 years. Among these patients, there were 94 (63.9%) female and 53 (36.1%) male with no significant (p>0.05) sex difference between the T2D and ND patients. The age and FBS were significantly higher (p<0.05) in the T2D patients compared to ND patients while the weight, height; SBP and DBP were not significant [Table/Fig-1].
Baseline characteristics of the study participants.
| Characteristics | T2D | ND | Minimum | Maximum | p-value |
|---|
| Age (years) | 56.83±1.21 | 49.03±1.89 | 30 | 92 | 0.001 |
| Height (m) | 1.58±0.01 | 1.61±0.01 | 1.37 | 1.90 | 0.179 |
| Weight (kg) | 78.85±3.42 | 71.52±1.95 | 35.00 | 190.00 | 0.063 |
| SBP (mmHg) | 132.69±2.65 | 132.86±3.23 | 100 | 213 | 0.967 |
| DBP (mmHg) | 78.80±1.36 | 81.76±2.24 | 58 | 151 | 0.240 |
| FBS (mg/dL) | 166.71±11.39 | 65.75±3.79 | 11.00 | 520.00 | <0.001 |
Legend: Results are presented as Mean±SEM- SE: Standard Error of the mean; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; FBS: Fasting Blood Sugar
Serum Electrolytes and Renal Function Indices: The serum electrolytes varied among the patients as summarised in [Table/Fig-2]. K+ level was significantly higher (p=0.002) in the T2D group (3.82±0.31 mEq/L) compared to the ND group (2.66±0.22 mEq/L). Na+ and Cl- level were also higher in the T2D compared to the ND patients but the differences were not significant (p>0.05). Similarly, the renal function indices; creatinine and urea, were higher in the T2D patients than in the ND patients but the differences were not significant (p>0.05).
Electrolytes and renal function indices in T2D and ND patients.
| Parameter | T2D (n = 72) | ND (n = 75) | Minimum | Maximum | p-value |
|---|
| Potassium (mEq/L) | 3.82±0.31 | 2.66±0.22 | 0.1 | 18.1 | 0.002 |
| Sodium (mEq/L) | 153.65±13.31 | 144.25±14.70 | 3.30 | 723.60 | 0.637 |
| Chorine (mEq/L) | 74.30±6.09 | 73.71±6.09 | 2.33 | 340.00 | 0.945 |
| Creatinine (mg/dL) | 1.77±0.42 | 1.21±0.11 | 0.02 | 29.34 | 0.192 |
| Urea (mg/dL) | 40.72±4.24 | 35.98±5.48 | 1.29 | 276.50 | 0.497 |
Derangement of Electrolytes and Renal Function Indices: [Table/Fig-3] summarises the derangement of electrolytes and renal function indices. More patients with T2D were hyperkalaemic (8.8%) than the ND patients (2.7%) while hypokalaemia was more present in the ND patients (41.5%) than in T2D patients (25.2%). As such, the proportion of hyperkalaemia and hypokalaemia was significantly different (p = 0.001) between T2D and ND patients. The proportion of hypernatraemia was insignificantly higher in T2D (15.6%) than in ND patients (15.0%). The proportion of hypochloraemia and hyperchloraemia were both similar in the T2D and ND patients without any significant difference (p = 0.494). Also, the proportion of hypercreatinaemia and hyperureaemia were higher in T2D compared to ND patients though not significantly (p>0.05).
Proportion of T2D and ND patients with electrolytes and renal function indices derangement.
| Parameter | Category | T2D (%) (n =72) | ND (%) (n = 75) | Total (%) (n =147) | χ2 | r | p-value |
|---|
| Potassium (mEq/L) | Hypok-alaemia | 37 (25.2%) | 61 (41.5%) | 98 (66.7%) | 15.087 | 0.320 | 0.001 |
| Normal | 22 (15.0%) | 10 (6.8%) | 32 (21.8%) |
| Hyperk-alaemia | 13 (8.8%) | 4 (2.7%) | 17 (11.6%) |
| Sodium (mEq/L) | Hypona-traemia | 39 (26.5%) | 50 (34.0%) | 89 (60.5%) | 5.092 | 0.186 | 0.078 |
| Normal | 10 (6.8%) | 3 (2.0%) | 13 (8.8%) |
| Hyperna-traemia | 23 (15.6%) | 22 (15.0%) | 45 (30.6%) |
| Chloride (mEq/L) | Hypoch-loraemia | 59 (40.1%) | 65 (44.2%) | 124 (84.4%) | 1.412 | 0.098 | 0.494 |
| Normal | 1 (0.7%) | 0 (0.0%) | 1 (0.7%) |
| Hyperch-loraemia | 12 (8.2%) | 10 (6.8%) | 22 (15.0%) |
| Creatinine (mg/dL) | Low | 20 (13.6%) | 16 (10.9%) | 36 (24.5%) | 2.708 | 0.136 | 0.439 |
| Normal | 16 (10.9%) | 25 (17.0%) | 41 (27.9%) |
| High | 34 (23.1%) | 33 (22.4%) | 67 (45.6%) |
| Very High | 2 (1.4%) | 1 (0. 7%) | 3 (2.0%) |
| Urea (mg/dL) | Hypou-raemia | 8 (5.4%) | 14 (9.5%) | 22 (14.9%) | 2.877 | 0.140 | 0.411 |
| Normal | 47 (32.0%) | 46 (31.3%) | 93 (63.3%) |
| Hyperu-raemia | 17 (11.6%) | 14 (9.5%) | 31 (21.1%) |
| ND | 0 (0.0%) | 1 (0.7%) | 1 (0.7%) | | | |
Association between Study Parameters
Association between study parameters is summarised in [Table/Fig-4]. Most serum electrolytes and renal function indices insignificantly (p>0.05) correlated positively with age of patients except for K+ and Na+ which were significant (p<0.05). SBP significantly correlated positively (p=0.039) with Cl- but insignificantly (p>0.05) with Na+ while it insignificantly correlated negatively with K+, urea and creatinine. DBP negatively correlated with K+ significantly and insignificantly with creatinine while Na+, Cl- and urea showed positive correlations which were insignificant. FBS positively correlated with Cl-, creatinine and urea insignificantly but significantly (p=0.04) with K+ while the correlation with Na+ was negative. As shown in [Table/Fig-5], urea insignificantly correlated positively (p>0.05) with K+, Na+ and Cl- while creatinine level positively correlated significantly with K+ (p=0.004) but insignificantly with Na+ and Cl- (p>0.05).
Association of age, SBP, DBP and FBS with electrolyte and renal function indices.
| Parameter | Potassium ion | Sodium ion | Chloride ion | Creatinine | Urea |
|---|
| r | p-value | r | p-value | r | p-value | r | p-value | r | p-value |
| Age | 0.214 | 0.010 | 0.160 | 0.050 | 0.050 | 0.552 | 0.022 | 0.794 | 0.027 | 0.752 |
| Systolic Blood Pressure | -0.151 | 0.111 | 0.126 | 0.181 | 0.194 | 0.039 | -0.104 | 0.270 | -0.039 | 0.682 |
| Diastolic Blood Pressure | -0.180 | 0.050 | 0.020 | 0.830 | 0.046 | 0.629 | -0.058 | 0.541 | 0.013 | 0.890 |
| FBS | 0.173 | 0.040 | -0.015 | 0.855 | 0.023 | 0.785 | 0.037 | 0.663 | 0.100 | 0.238 |
r: Pearson Correlation coefficient; FBS: Fasting Blood Sugar; figures in bold indicate significant difference (p<0.05)
Association between electrolytes and renal function indices.
| Parameters | Potassium ion | Sodium ion | Chloride ion |
|---|
| r | p-value | r | p-value | r | p-value |
| Creatinine | 0.235 | 0.004 | 0.065 | 0.438 | 0.130 | 0.118 |
| Urea | 0.063 | 0.449 | 0.071 | 0.391 | 0.126 | 0.131 |
r: Pearson Correlation coefficient; figures in bold indicate significant difference (p<0.05)
Discussion
Electrolyte disorders may occur as a result of hyperglycaemia, ketoacidosis, renal dysfunction as well as administration of drugs such as diuretics, antidiabetic agents or exogenous insulin etc. altering the electrolyte concentration in the body [25-27]. In this study, serum K+ level significantly increased (p< 0.05) in T2D patients compared to ND patients while the increase in Na+ level was not significant (p>0.05). These results are consistent with previously reported studies which have shown Na+ and K+ levels to increase in diabetic patients as a result of excessive loss of water due to osmotic diuresis [28,29]. On the other hand, Cl- was similar among the T2D and ND patients with no significant difference (p>0.05). This finding has also been observed in a previous study in which serum Cl- was shown not to be affected by hyperglycaemia [30].
Serum creatinine, and urea as well as albumin in urine are markers of diabetes nephropathy [31,32]. High creatinine and urea in serum are as a result of reduced glomerular filtration while high albumin in urine is as a result of impaired renal function. Elevated levels of these markers are evident when the glomerulus of the kidney becomes destroyed, leaks fluid and proteins due to prolong high blood glucose level in diabetics causing renal damage [33,34]. Serum creatinine as well as urea levels were higher in T2D patients compared to ND patients, though not significantly (p> 0.05). The high creatinine and urea levels in T2D subjects suggest that prolong high blood sugar can impair renal function thus, leading to accumulation of waste products and promotes renal dysfunction as previously confirmed [35-37].
Hypokalaemia is usually common in diabetes due to osmotic drift of fluid from the intracellular to extracellular spaces diluting K+ concentration [38]. In this study, the proportion of hyperkalaemia was higher in T2D than in ND patients confirming previous findings which have shown hyperkalaemia to be prominent in diabetics than in normal individuals [38]. The proportion of hyponatraemia was higher than hypernatraemia in T2D patients confirming the dominance of dilutional effect in a hyperglycaemic state [39,40]. Dysnatraemia in diabetes has been attributed to endocrine dysfunction whereby, hyperglycaemia can impair insulin and glucagon action [41]. Hyperchloraemia was more present in T2D patients than ND patients which could be due to hypertonicity although, hyperglycaemia has not been shown to have a significant impact on Cl-. The proportion of high and very high levels of creatinine (> 4 mg/dL), a marker for renal failure as well as hyperuraemia was higher in T2D patients compared to ND patients though not significantly (p> 0.05).
Certain factors such as age, high blood pressure, obesity etc. are known to confer risk in the development of T2D [42]. Since T2D is characterised by hyperglycaemia and known to cause electrolyte imbalance as well as diabetic nephropathy, some of these factors were evaluated for possible association with serum electrolyte as well as renal function indices [43]. K+ and Na+ showed significant (p<0.05) positive association with age suggesting older people are more prone to have high levels of K+ and Na+ in the body. K+ correlated negatively with DBP while Cl- correlated positively with SBP significantly (p<0.05). However, Na+ as well as creatinine and urea did not correlate with SBP and DBP. FBS positively correlated with K+ thus high blood sugar can lead to high K+ levels. However, this is in contrast to the general scenario where hyperglycaemia is known to cause hypokalemia in most cases [44].
Renal damage could also lead to electrolyte derangement especially when the glomerulus is destroyed and leaks out fluid [45]. Since creatinine and urea are indicative of progression of renal damage, increase in the levels of these markers may influence the serum electrolyte levels [46]. Creatinine was found to be associated positively with K+, implying increase in creatinine which is indicative of possible renal damage, could influence an increase in K+ level in serum. As such, prolong hyperglycaemia observed in diabetes may lead to electrolytes derangement either directly through osmotic drift or indirectly through progression of diabetic nephropathy.
Conclusion
Findings from this study reveal T2D to promote electrolyte disorder and may possibly influence the renal function. Also, renal dysfunction characterised by elevated creatinine and urea may promote electrolyte derangement, especially potassium ion disorder. Therefore, serum electrolyte disorders may be as a result of hyperglycaemia as well as renal dysfunction. Moreover, old age and high blood pressure are possible risk factors of serum electrolyte disorders.
Conflict of interest
The authors stated that they have no conflicts of interest regarding the publication of this article.
Legend: Results are presented as Mean±SEM- SE: Standard Error of the mean; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; FBS: Fasting Blood Sugar
[1]. Lobo DN, Fluid, electrolytes and nutrition: physiological and clinical aspectsProc Nutr Soc 2004 63(3):453-66.10.1079/PNS200437615373958 [Google Scholar] [CrossRef] [PubMed]
[2]. Liamis G, Rodenburg EM, Hofman A, Zietse R, Stricker BH, Hoorn EJ, Electrolyte disorders in community subjects: prevalence and risk factorsAm J Med 2013 126:256-63.10.1016/j.amjmed.2012.06.03723332973 [Google Scholar] [CrossRef] [PubMed]
[3]. Palmer BF, Managing hyperkalaemia caused by inhibitors of the renin-angiotensinaldosterone systemNew Engl J Med 2004 351:585-92.10.1056/NEJMra03527915295051 [Google Scholar] [CrossRef] [PubMed]
[4]. Sotirakopoulos N, Kalogiannidou I, Tersi M, Armentzioiou K, Sivridis D, Mavromatidis K, Acid–base and electrolyte disorders in patients with diabetes mellitusSaudi J Kidney Dis Transplant 2012 23(1):58-62. [Google Scholar]
[5]. Liamis G, Liberopoulos E, Barkas F, Elisaf M, Spurious electrolyte disorders: a diagnostic challenge for cliniciansAm J Nephrol 2013 38:50-57.10.1159/00035180423817179 [Google Scholar] [CrossRef] [PubMed]
[6]. Liamis G, Liberopoulos E, Barkas F, Elisaf M, Diabetes mellitus and electrolyte disordersWorld J Clin Cases 2014 2(10):488-96.10.12998/wjcc.v2.i10.48825325058 [Google Scholar] [CrossRef] [PubMed]
[7]. DeFronzo RA, Goldberg M, Agus ZS, The effects of glucose and insulin on renal electrolyte transportJ Clin Invest 1976 58(1):83-90.10.1172/JCI108463932211 [Google Scholar] [CrossRef] [PubMed]
[8]. Palmer BF, Clegg DJ, Electrolyte and acid–base disturbances in patients with diabetes mellitusNew Engl J Med 2015 373(6):548-59.10.1056/NEJMra150310226244308 [Google Scholar] [CrossRef] [PubMed]
[9]. Uribarri J, Oh MS, Carroll HJ, Hyperkalemia in diabetes mellitusJ Diabetes Complications 1990 4:03-07.10.1016/0891-6632(90)90057-C [Google Scholar] [CrossRef]
[10]. Liamis G, Tsimihodimos V, Doumas M, Spyrou A, Bairaktari E, Elisaf M, Clinical and laboratory characteristics of hypernatraemia in an internal medicine clinicNephrol Dialysis Transplant 2008 23:136-43.10.1093/ndt/gfm37617932111 [Google Scholar] [CrossRef] [PubMed]
[11]. Wang S, Hou X, Liu Y, Lu H, Wei L, Bao Y, Serum electrolyte levels in relation to macrovascular complications in Chinese patients with diabetes mellitusCardiovasc Diabetol 2013 12:14610.1186/1475-2840-12-14624112518 [Google Scholar] [CrossRef] [PubMed]
[12]. Liamis G, Milionis H, Elisaf M, A review of drug-induced hyponatremiaAm J Kidney Dis 2008 52:144-53.10.1053/j.ajkd.2008.03.00418468754 [Google Scholar] [CrossRef] [PubMed]
[13]. Sarguru D, Vanaja R, Balaji R, Evaluation of serum electrolytes in type II diabetes mellitusInt J Pharm Sci Rev Res 2016 40(1):251-53. [Google Scholar]
[14]. Steyn NP, Mann J, Bennett PH, Temple N, Zimmet P, Tuomilehto J, Diet, nutrition and the prevention of type 2 diabetesPub Hlth Nutr 2004 7(1A):147-65.10.1079/PHN200358614972058 [Google Scholar] [CrossRef] [PubMed]
[15]. International Diabetes Federation. International Working Group on the Diabetic Foot. http://www.idf.org/webdata/docs/background_info_AFR.pdf. 2016, Accessed 5/5/17. [Google Scholar]
[16]. Charan J, Biswas T, How to calculate sample size for different study designs in medical Research?Indian J Psychol Med 2013 35:121-26.10.4103/0253-7176.11623224049221 [Google Scholar] [CrossRef] [PubMed]
[17]. Chen S, Lansdown AJ, Moat SJ, Ellis R, Goringe A, Dunstan FDJ, An observational study of the effect of metformin on B12 status and peripheral neuropathyBr J Diabetes Vascular Dis 2012 12:189-93.10.1177/1474651412454924 [Google Scholar] [CrossRef]
[18]. WHO-IDF. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. http://www.idf.org/webdata/docs/WHO_IDF_definition_diagnosis_of_diabetes.pdf 2014, Accessed 1/10/2014 [Google Scholar]
[19]. Skeggs L, Hochstrasser H, Multiple automatic sequential analysisClin Chem 1964 10:918-36. [Google Scholar]
[20]. Maruna RF, Determination of serum sodium by the magnesium uranyl acetateClin Chem Acta 1958 2:580-84. [Google Scholar]
[21]. Trinder PA, Rapid method for the determination of sodium in serumAnalyst 1951 76:596-99.10.1039/an9517600596 [Google Scholar] [CrossRef]
[22]. Schoenfeld RG, Lewellen CJ, A colorimetric method for the determination of serum chloride, ClinChem 1964 10:533-39. [Google Scholar]
[23]. Watt GW, Crisp JD, Spectrophotometric method for determination of ureaAnalytical Chem 1954 26:452-53.10.1021/ac60087a006 [Google Scholar] [CrossRef]
[24]. Owen A, Iggo B, Scandrett FJ, Stewart CP, The determination of creatinine in plasma or serum and in urine: A critical examinationBiochem J 1954 58:426-30.10.1042/bj058042613208633 [Google Scholar] [CrossRef] [PubMed]
[25]. Al-Rubeaan K, Siddiqui K, Abu Risheh K, Hamsirani R, Alzekri A, Alaseem A, Correlation between serum electrolytes and fasting glucose and Hb1Ac in Saudi diabetic patientsBiol Trace Element Res 2011 144(1–3):463-68.10.1007/s12011-011-9144-421818670 [Google Scholar] [CrossRef] [PubMed]
[26]. Chiasson JL, Aris-Jilwan N, Bélanger R, Bertrand S, Beauregard H, Ekoé JM, Diagnosis and treatment of diabetic ketoacidosis and the hyperglycemic hyperosmolar stateCan Med Assoc J 2003 168:859-66. [Google Scholar]
[27]. Moses AM, Howanitz J, Miller M, Diuretic action of three sulfonylurea drugsAnn Int Med 1973 78:541-44.10.7326/0003-4819-78-4-5414632790 [Google Scholar] [CrossRef] [PubMed]
[28]. Syed MS, Roomana R, Tabassum M, Electrolytes and sodium transport mechanism in diabetes mellitusPak J Pharm Sci 2005 18(2):06-10. [Google Scholar]
[29]. Lindner G, Funk GC, Hypernatremia in critically ill patientsJ Crit Care 2013 28:216.e11-20.10.1016/j.jcrc.2012.05.00122762930 [Google Scholar] [CrossRef] [PubMed]
[30]. Deepti GN, Sumina C, Lakshmi K, A comparative study of electrolyte imbalances in controlled and uncontrolled diabetes mellitusInt J Clin Biochem Res 2017 4(1):22-24. [Google Scholar]
[31]. Anjaneyulu M, Chopra K, Quercetin, an antioxidant bioflavonoid, attenuates diabetic nephropathy in ratsClin Exp Pharmacol Physiol 2004 31(4):244-48.10.1111/j.1440-1681.2004.03982.x15053821 [Google Scholar] [CrossRef] [PubMed]
[32]. Schena FP, Gesualdo L, Pathogenetic mechanisms of diabetic nephropathyJ Am Soc Nephrol 2005 16(3):S30-33.10.1681/ASN.200411097015938030 [Google Scholar] [CrossRef] [PubMed]
[33]. Judykay T, Nutrition for reducing urea and creatinine in the bloodDiabetes Care 2007 27:2191-92. [Google Scholar]
[34]. Siva L, Mythili SV, Kumar PS, Biochemical and haematological aberrations in type I and type II diabetic patients in South India: a comparative studyInt J Res Pharm Biochem Sci 2012 3(2):22-29. [Google Scholar]
[35]. Sugam S, Prajwal G, Rojeet S, Bibek P, Manoj S, Prashant R, Serum urea and creatinine in diabetic and non-diabetic subjectsJ Nepal Assoc Med Lab Sci 2008 9(1):11-12. [Google Scholar]
[36]. Idonije BO, Oloruntoba F, Olarewaju MO, Plasma glucose, creatinine and urea levels in type 2 diabetes patients attending a Nigerian teaching hospitalRes J Med Sci 2011 5(1):01-03.10.3923/rjmsci.2011.1.3 [Google Scholar] [CrossRef]
[37]. Mishra KP, Mawar A, Kare PK, Verma N, Relationship between fasting blood glucose, serum urea, serum creatinine and duration of diabetes in type-2 diabetic patientsFlora and Fauna 2015 21(1):127-32. [Google Scholar]
[38]. Elisaf MS, Tsatsoulis AA, Katopodis KP, Siamopoulos KC, Acid-base and electrolyte disturbances in patients with diabetic ketoacidosisDiabetes Res Clin Pract 1996 34:23-27.10.1016/S0168-8227(96)01332-0 [Google Scholar] [CrossRef]
[39]. Roscoe J, Halperin M, Rolleston F, Goldstein M, Hyperglycemia-induced hyponatremia: metabolic considerations in calculation of serum sodium depressionCan Med Assoc J 1975 112(4):452-53. [Google Scholar]
[40]. Liamis G, Milionis HJ, Elisaf M, Hyponatremia in patients with infectious diseasesJ Infect 2011 63:327-35.10.1016/j.jinf.2011.07.01321835196 [Google Scholar] [CrossRef] [PubMed]
[41]. Bratusch-Marrain PR, DeFronzo RA, Impairment of insulin mediated glucose metabolism by hyperosmolality in manDiabetes 1983 32:1028-34.10.2337/diab.32.11.10286416909 [Google Scholar] [CrossRef] [PubMed]
[42]. Yufang B, Tiange W, Min X, Yu X, Mian L, Jieli L, Advanced research on risk factors of type 2 diabetesDiabetes Met Res Rev 2012 28(2):32-39.10.1002/dmrr.235223280864 [Google Scholar] [CrossRef] [PubMed]
[43]. O’Bryan GT, Hostetter TH, The renal hemodynamic basis of diabetic nephropathySemin Nephrol 1997 17:93-100. [Google Scholar]
[44]. Raebel MA, Ross C, Xu S, Roblin DW, Cheetham C, Blanchette CM, Diabetes and drug-associated hyperkalemia: effect of potassium monitoringJ Gen Internal Med 2010 25:326-33.10.1007/s11606-009-1228-x20087674 [Google Scholar] [CrossRef] [PubMed]
[45]. Molitch ME, Defronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, The American Diabetes Association: Nephropathy in diabetes (Position Statement)Diabetes Care 2004 27(1):S79-S83.10.2337/diacare.27.2007.S7914693934 [Google Scholar] [CrossRef] [PubMed]
[46]. Sirwal IA, Banday KA, Reshi AR, Bhat MA, Wani MM, Estimation of Glomerular Filteration Rate (GFR)JK Sci 2004 6:121-23. [Google Scholar]