Diabetes Mellitus (DM) is the most common metabolic disorder worldwide affecting 6-10% of the population [1]. Efficient management and appropriate treatment is required to achieve good diabetes control in order to reduce the risk of diabetic complications [1].
Glycemic control can be evaluated by measurement of glycated haemoglobin (HbA1c) and Self-Monitoring of Blood Glucose (SMBG) [2]. Both are equally important; HbA1c depicts the average Blood Glucose (BG) levels over the last three months, while SMBG values evaluate the individuals’ response to therapy and allow adjustment of antidiabetic treatment [2]. However, HbA1c measurement has several limitations. Firstly, a laboratory satisfying the established requirements for HbA1c measurement is required [3]. Moreover, conditions like chronic kidney disease, haemoglobinopathies and iron deficiency impact HbA1c reliability [3,4]. Furthermore, HbA1c does not provide a measure of glycaemic variability, which is an independent risk factor for diabetic complications [5]. The number of blood measurements depends on several parameters, such as type of diabetes, antidiabetic treatment and glycaemic targets. Patients who measure BG levels more frequently have lower HbA1c [6,7].
Regarding the importance of self-measurement, SMBG systems need to fulfill some minimum performance criteria in order to be accurate and safe for use [8]. Such requirements were established in 2003 and revised in 2013 (ISO 15197:2013) [9,10]. The revised criteria require that 95% of the individual BG results must be within ±15mg/dL of the comparison-method results at BG concentrations <100mg/dL and within ±15% of the comparison-method results at BG concentrations ≥100mg/dL. Moreover, consensus error grid analyses should be performed to determine the clinical relevance of the accuracy of BG measurements [11]. This was first established in 2000 and was included in the ISO guidelines [10,11].
GM700S (Bionime, Berneck, Switzerland), OneTouch Verio IQ (LifeScan, Chesterbrook, PA, USA), Freestyle Optium Neo (Abbott Diabetes Care, North Chicago, IL, USA), Contour NEXT (Ascensia Diabetes Care, Parsippany, NJ, USA), Accu–Chek Aviva Plus (Roche Diabetes Care Inc., Indianapolis, IN, USA) and Glucomen Areo (A. Menarini Diagnostics, FIorence, Italy) are SMBG systems that are widely available in Greece. Previous studies have shown that most of them fulfill the ISO requirements [12-17]. Nevertheless, apart from the ISO requirements; usability of glucometers is an important factor to be considered. However, data on the patient satisfaction of SMBG systems are scarce.
The aim of the study was to evaluate the accuracy performance in requirements resembling the ISO 15197:2013 and the usability of six commonly used SMBG systems.
Materials and Methods
i. Participants: This cross-sectional study took place for one week in July of 2016 in the Diabetes Center of Laiko General Hospital. A total of 120 patients with DM were recruited consecutively from the outpatient Diabetes Clinic since the ISO 15197:2013 require the evaluation of at least 100 different subjects [10]. Adults 18-80 years of age with DM were included in the study. Diagnosis of DM was based on the criteria adopted from the American Diabetes Association [3]. Patients that were diagnosed with serious complications in the previous six months, such as myocardial infarction, serious trauma and major surgery, individuals with mental illness, severe anaemia (haematocrit lower than 30%) or pregnant women were excluded. The purpose of the study was clearly explained in written to all subjects, who then volunteered to participate. All patients gave their written informed consent before participation in the study, which was conducted according to the principles of the Declaration of Helsinki [18]. The ethics committee of our hospital approved the study.
ii. Laboratory Analyses: All procedures were carried out in the morning in an environment of stable temperature (23±5°C) with a humidity range of the environment between 20% and 90% based on the manufacturers’ instruction for use. The finger skin was punctured using a disposable lancet and fresh capillary blood samples were collected into micro-tubes with lithium heparin anticoagulant by the study personnel (about 600 μL of fresh whole blood was collected) as described previously [12,14,19].
The sample was then applied on the six different glucometers that are frequently used in Greece (GM700S, OneTouch Verio IQ, Freestyle Optium Neo, Contour NEXT, Accu–Chek Aviva Plus and Glucomen Areo), according to the manufacturers’ instructions. For each glucometer, two test strip lots were used in each sample. The order of testing was alternated among the SMBG devices and the YSI 2300 STAT PLUS (YSI Incorporated, Yellow Springs, Ohio, USA) in a predefined rotation. The rotation was performed to ensure that the test order would not affect the BG results. All strips had already been inserted in the different meters before blood drop was placed. The mean of BG measurements from each SMBG device were compared with the mean of duplicate results obtained from the YSI 2300 STAT PLUS. The mean time from sample collection to glucose measurements was less than 7 minutes, as described previously [14].
Participants were punctured for a second time at the finger and the blood was collected in a Micro Haematocrit capillary tube (Kimble Chase, Rockwood, TN, USA). Blood was then centrifuged and a ruler was used to measure the length of the column of the packed red cells, which was then divided by the length of the column of the whole blood and multiplied by 100% to obtain the haematocrit of the blood sample, as descried previously [12].
According to the recommendation of the ISO 15197:2013, a wide BG concentration needs to be tested for each SMBG system. More specifically, 5% of the tested glucose samples should have BG values ≤50mg/dL, 15% between 51-80mg/dL, 20% between 81-120mg/dL, 30% between 121-200mg/dL, 15% between 201-300mg/dL, 10% between 301-400mg/dL and 5% of the glucose samples should have BG values >400mg/dL [10]. If the collected samples did not provide a sufficient percentage of the required BG range, samples over 400mg/dL were artificially produced in the laboratory using glucose solution, while samples below 50mg/dL were produced by incubating blood samples at room temperature until BG concentration reached low levels because of glucose consumption by erythrocytes, as described previously [10,20]. The Partial Pressure of Oxygen (po2) in adjusted blood samples was checked (OptiTM Check; OPTI Medical Systems Inc., Roswell, GA) in order to ensure a po2 that is comparable to the po2 in capillary blood, as described previously [19].
At the end of the visit patients had enough time to process and use the glucometers and perform BG measurement by themselves. Then, they were asked to complete a Likert Scale Questionnaire about the size of the glucometers, the blood sample application on the test strip, the insertion of the test strip on the meter, the size of the indication on the screen and the removal of the test strip.
iii. Reference Method: YSI 2300 STAT PLUS uses fresh venous or capillary whole blood. Measurements are performed using glucose oxidase as an assay method. For our study, quality control was performed for every five measurements, as described previously [12].
iv. Statistical Analysis: SMBG systems accuracy was assessed by comparison of the mean of the two BG measurements obtained with each SMBG device with the respective mean of the two glucose measurements obtained with the reference method. At BG concentrations <100mg/dL, the absolute and relative number Consequencesof each glucometer results within ±15mg/dL, ±10mg/dL, and ±5mg/dL of the comparison measurement was calculated. At BG concentrations ≥100mg/dL, the absolute and relative number of each SMBG system results within ±15%, ±10%, and ±5% of the comparison measurement was calculated. The agreement between each SMBG system measurement and the reference method result was plotted in a difference-plot as recommended in DIN EN ISO 15197:2013 [10].
Consensus error grid was used for evaluating the clinical relevance of the result between the BG measurement by SMBG device and the BG measurement by the reference method [10,11]. The five zones and their clinical importance are shown in [Table/Fig-1].
Definitions of the error grid zones.
| Risk level (CEG zone) | Definition of risk level | Risk to patient with diabetes |
|---|
| A | SMBG <20% deviation from true BG or both SMBG and BG <70mg/dL | No effect on clinical outcome |
| B | Deviation from true BG >20% | Little or no effect on clinical outcome |
| C | Overcorrection of acceptable BG levels | Likely to affect the clinical outcome |
| D | Dangerous failure to detect and treat BG errors | Significant medical impact |
| E | Treatment contradictory to that actually required | Dangerous Consequences |
CEG: Consensus Error Grid, SMBG: Self-Monitoring Blood Glucose, BG: Blood Glucose
Results
i. Participants: A total of 120 participants were recruited. The characteristics of the study participants are shown in [Table/Fig-2]. The haematocrit range was 30.8 to 52.9% and the glucose range was 38.7 to 475mg/dL. Regarding the distribution of BG concentrations of the blood samples collected, 6 (5.0%) had BG concentration <50mg/dL, 20 (16.7%) between 50 and 80mg/dL, 23 (19.2%) between 80 and 120mg/dL, 37 (30.8%) between 120 and 200mg/dL, 16 (13.3%) between 200 and 300mg/dL, 12 (10.0%) between 300 and 400mg/dL, and 6 (5.0%) had BG levels >400mg/dL.
Characteristics of the study participants.
| Gender n (%) | Age (years) n (%) | Type of diabetes n (%) | Diabetes duration (years) n (%) | SMBG use (years) n (%) | Education level n (%) |
|---|
| Male | 67 (55.8) | 18-20 | 2 (1.7) | Type 1 | 21 (17.5) | 0-3 | 22 (18.3) | 0-3 | 25 (20.8) | Elementary school | 17 (14.0) |
| Female | 53 (44.2) | 21-30 | 5 (4.2) | Type 2 | 95 (79.2) | 4-6 | 21 (17.5) | 4-6 | 20 (16.6) | Middle school | 28 (23.0) |
| | 31-40 | 7 (5.8) | Unknown | 4 (3.3) | 7-9 | 9 (7.5) | 7-9 | 9 (7.5) | High school | 41 (34.0) |
| | 41-50 | 17 (14.2) | | | 10-15 | 25 (20.8) | 10-15 | 24 (20.0) | Graduate school | 33 (28.0) |
| | 51-60 | 24 (20.0) | | | 16-20 | 15 (12.5) | 16-20 | 14 (11.7) | College | 0 (0) |
| | 61-70 | 39 (32.5) | | | 21-24 | 5 (4.2) | 21-24 | 6 (5.0) | No answer | 1 (1.0) |
| | 71-80 | 26 (21.7) | | | >25 | 21 (17.5) | >25 | 20 (16.7) | | |
| | | | | | Unknown | 2 (1.7 | Unknown | 2 (1.7 | | |
SMBG: Self-Monitoring Blood Glucose
ii. Accuracy: Above 95% of the results of GM700S, Contour NEXT, Accu-Chek Aviva Plus and OneTouch Verio IQ in both BG levels <100 mg/dL and ≥100 mg/dL were within the required limits. A total of 95.0% of the results of Glucomen Areo were within the required limits in all BG concentration, but only 91.3% of the results in BG levels <100 mg/dL were within the required limits. Less than 95% of the results of Freestyle Optium Neo were within the required limits in all BG concentration. The accuracy plots are shown in [Table/Fig-3]. When more strict criteria where used, the glucometer with the highest percentage of results within the required limits was GM700S [Table/Fig-4].
Self-monitoring blood glucose systems accuracy plots: a: GM700S, b: OneTouch Verio IQ, c: Freestyle Optium Neo, d: Contour NEXT, e: Accu-Chek Aviva Plus, f: Glucomen Areo.
The black lines represent the ±15 mg/dL / ±15% limits recommended by the ISO 15197:2013 requirements.
* The difference between each individual measured value from the SMBG system and the measured value from the reference method (YSI 2300 STAT PLUS).
SMBG: Self-Monitoring Blood Glucose
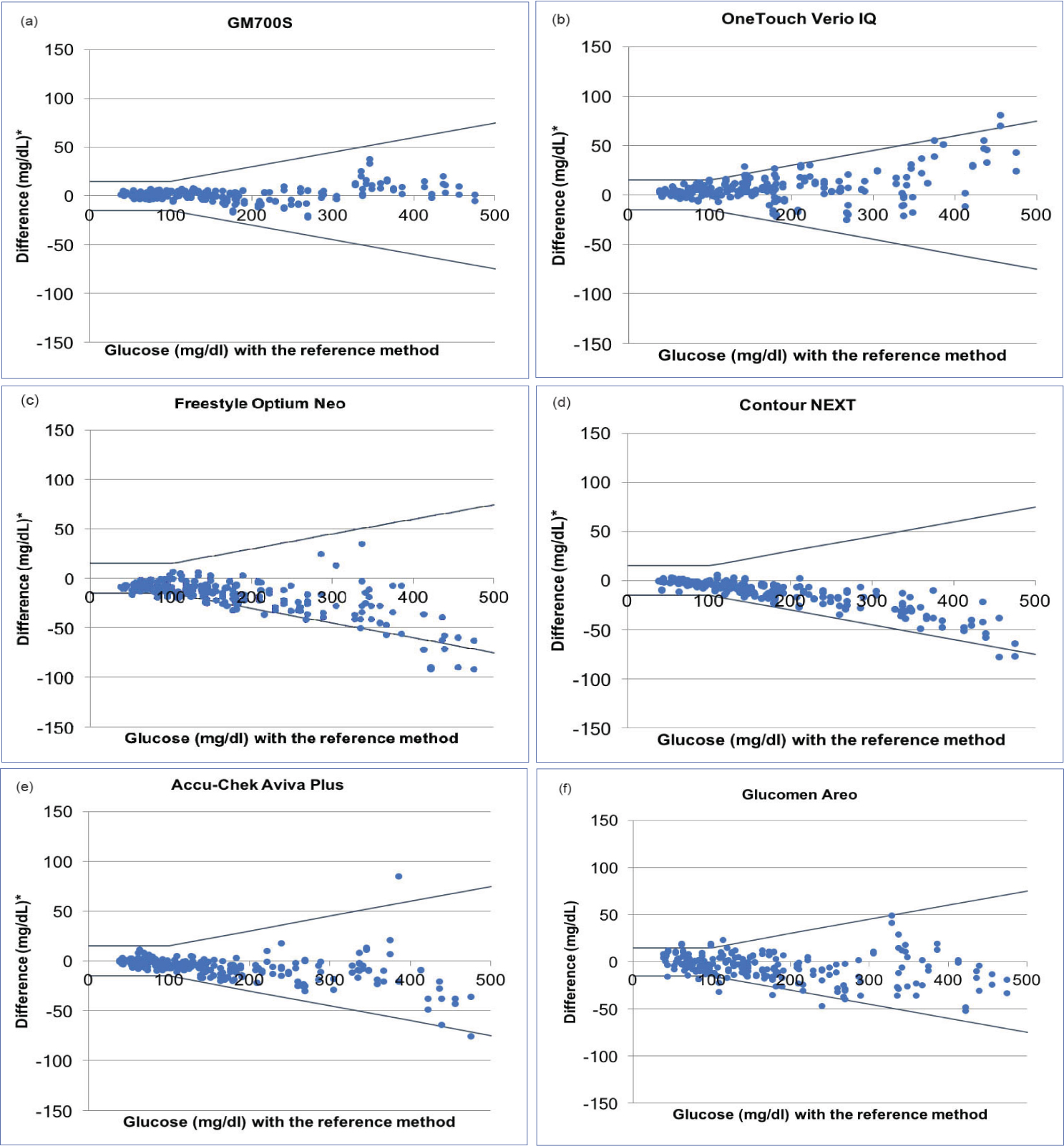
SMBG systems accuracy according to ISO 15197:2013 and to tighter requirements.
| BG < 100 mg/dL (n=40) | BG ≥ 100 mg/dL (n=80) | All BG range (n=120) |
|---|
| ≤ ±15 mg/dL | ≤ ±10 mg/dL | ≤ ±5 mg/dL | ≤ ±15 % | ≤ ±10 % | ≤ ±5 mg/dL | ≤ ±15 mg/dL or ±15 % | ≤ ±10 mg/dL |
| GM700S | 80/80* 100.0% | 80/80 100.0% | 71/80 88.8% | 160/160 100.0% | 159/160 99.4% | 142/160 88.8% | 240/240 100.0% | 239/240 99.6% |
| OneTouch Verio IQ | 79/80 98.8% | 72/80 90.0% | 53/80 66.3% | 156/160 97.5% | 131/160 82.9% | 82/160 51.3% | 235/240 97.9% | 204/240 85.0% |
| FreeStyle Optium Neo | 74/80 92.5% | 49/80 61.3% | 17/80 21.3% | 136/160 85.0% | 91/160 56.9 % | 32/160 20.0% | 210/240 87.5% | 140/240 58.3% |
| Contour NEXT | 80/80 100.0% | 78/80 97.5% | 71/80 88.8% | 158/160 98.8% | 126/160 78.8% | 51/160 31.9% | 238/240 99.2% | 204/240 85.0% |
| Accu-Chek Aviva Plus | 80/80100.0% | 79/80 98.8% | 58/80 72.5% | 158/160 98.8% | 143/160 89.4% | 85/160 53.1% | 238/240 99.2% | 222/240 92.5% |
| Glucomen Areo | 73/80 91.3% | 64/80 80.0% | 38/80 47.5% | 155/160 96.9% | 127/160 79.4% | 79/160 49.4% | 228/240 95.0% | 191/240 79.6% |
*Blood measurements were performed twice for each patient; SMBG: self-monitoring blood glucose, BG: blood glucose
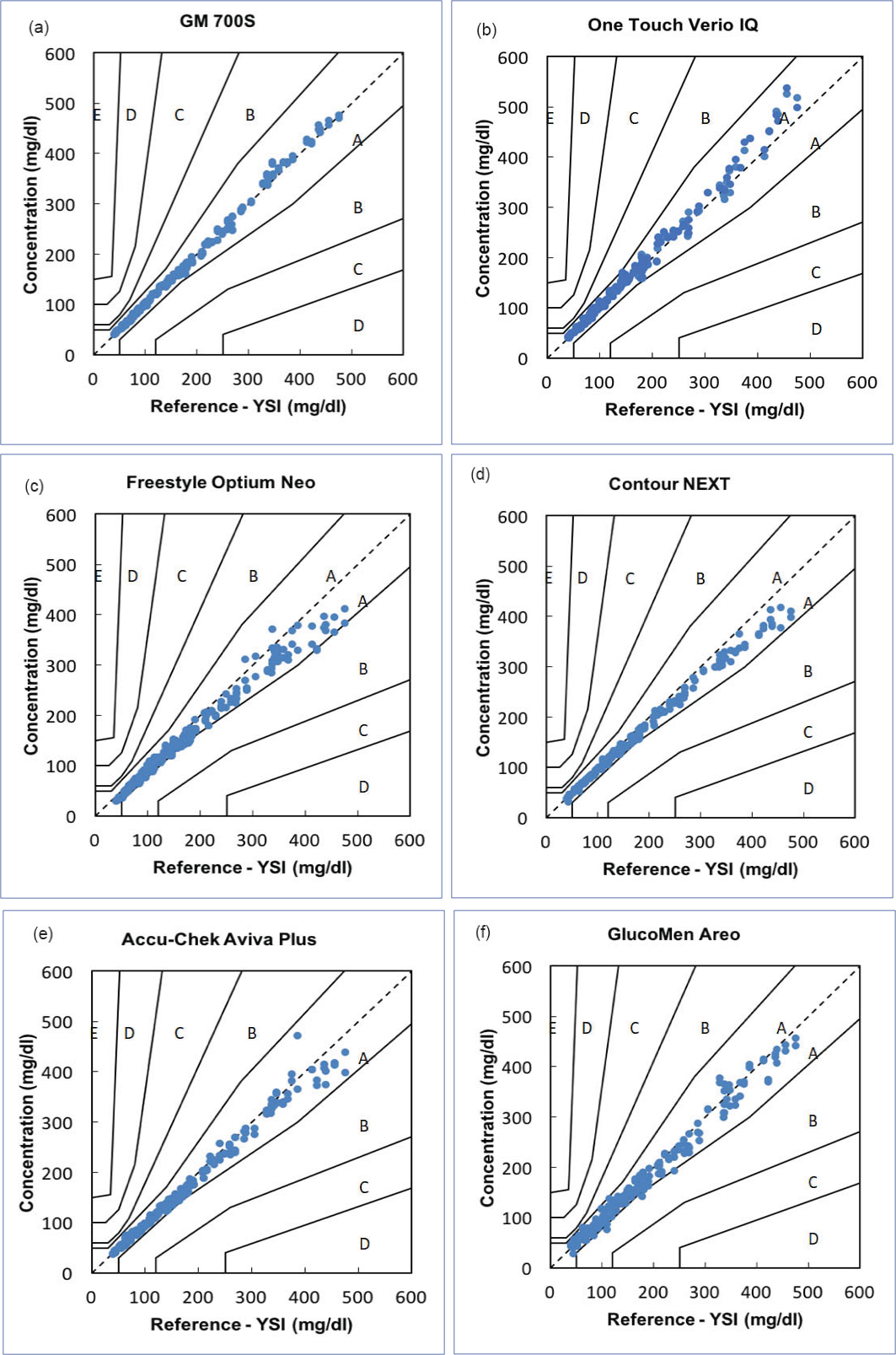
iii. Clinical Relevance: Consensus error grid analyses were performed for each SMBG system independently and are depicted in [Table/Fig-5]. All (100%) results obtained were within zone A and zone B signifying that the measurement error had no or little effect on clinical outcome.
Consensus error grid analysis for the results obtained with the 6 SMBG systems studied: a: GM700S, b: OneTouch Verio IQ, c: Freestyle Optium Neo, d: Contour NEXT, e: Accu-Chek Aviva Plus, f: Glucomen Areo
For each plot the y-axis represents the blood glucose result obtained by the SMBG system and the x-axis depicts the blood glucose result obtained by the reference method. The zones within each plot (A to E) indicate the increasing clinical significance of an erroneous measurement.
SMBG: Self-Monitoring Blood Glucose
iv. Usability: The results of the Likert Scale Questionnaire are shown in detail in [Table/Fig-6]. The one with the most suitable size among those tested was the One Touch Verio IQ followed by Contour NEXT. The SMBG device with the easiest blood sample application on the test strip was the Accu-Chek Aviva Plus, while the Contour Next was the second most convenient device. When patients were asked about the SMBG device with the clearest and most readable screen the FreeStyle Optium received the highest score. The SMBG device with the easiest strip insertion and the easier test strip removal was the Accu-Chek Aviva Plus [Table/Fig-6].
Participants’ Opinion (%) about the Usability of the SMBG Systems according to the Likert Scale Questionnaire.
| Question | Answer | Percentages of responses |
|---|
| GM7-00S | One Touch Verio IQ | FreeStyle Optium Neo | Contour NE XT | Accu-Chek Aviva Plus | GlucomenAreo |
|---|
| How do you feel about the size of the glucose meters? | Too large | 0.8% | 8.3% | 35.0% | 8.3% | 21.7% | 24.2% |
| Suitable | 80.8% | 86.7% | 63.3% | 82.5% | 74.2% | 70.0% |
| Too small | 18.3% | 5.0% | 0.8% | 8.3% | 2.5% | 5.8% |
| No comment | 0.0% | 0.0% | 0.8% | 0.8% | 1.7% | 0.0% |
| It was easy to apply your blood sample to the test strip. | Strongly agree | 5.8% | 10.8% | 18.3% | 25.8% | 27.5% | 10.8% |
| Agree | 60.8% | 72.5% | 75.8% | 70.8% | 64.2% | 82.5% |
| Disagree | 26.7% | 15.0% | 4.2% | 3.3% | 4.2% | 5.8% |
| Strongly disagree | 6.7% | 1.7% | 0.0% | 0.0% | 0.8% | 0.0% |
| No comment | 0.0% | 0.0% | 1.7% | 0.0% | 3.3% | 0.8% |
| It was easy to read the numbers/icons displayed on the LCD Screen. | Strongly agree | 14.2% | 24.2% | 32.5% | 28.3% | 25.8% | 16.7% |
| Agree | 77.5% | 71.7% | 64.2% | 65.0% | 67.5% | 73.3% |
| Disagree | 7.5% | 3.3% | 2.5% | 5.0% | 4.2% | 10.0% |
| Strongly disagree | 0.8% | 0.0% | 0.0% | 0.8% | 0.8% | 0.0% |
| No comment | 0.0% | 0.8% | 0.8% | 0.8% | 1.7% | 0.0% |
| It was easy to hold the strip and inserted the strip on the meter. | Strongly agree | 5.0% | 9.2% | 16.7% | 23.3% | 27.5% | 11.7% |
| Agree | 53.3% | 75.8% | 76.7% | 70.0% | 65.0% | 78.3% |
| Disagree | 29.2% | 13.3% | 5.8% | 5.8% | 5.8% | 9.2% |
| Strongly disagree | 12.5% | 1.7% | 0.0% | 0.8% | 0.0% | 0.0% |
| No comment | 0.0% | 0.0% | 0.8% | 0.0% | 1.7% | 0.8% |
| It was easy to remove used strips from the meter without touch blood sample. | Strongly agree | 34.2% | 16.7% | 35.8% | 17.5% | 40.0% | 23.3% |
| Agree | 44.2% | 69.2% | 59.2% | 60.0% | 52.5% | 65.0% |
| Disagree | 14.2% | 9.2% | 3.3% | 15.0% | 6.7% | 11.7% |
| Strongly disagree | 7.5% | 5.0% | 0.0% | 6.7% | 0.0% | 0.0% |
| No comment | 0.0% | 0.0% | 1.7% | 0.8% | 0.8% | 0.0% |
SMBG: Self-Monitoring Blood Glucose
Manufacturer details for each SMBG studied: GM700S (Bionime, Berneck, Switzerland), OneTouch Verio IQ (LifeScan, Chesterbrook, PA, USA), Freestyle Optium Neo (Abbott Diabetes Care, North Chicago, IL, USA), Contour NEXT (Ascensia Diabetes Care, Parsippany, NJ, USA), Accu–Chek Aviva Plus (Roche Diabetes Care Inc., Indianapolis, IN, USA) and Glucomen Areo (A. Menarini Diagnostics, FIorence, Italy).
Discussion
In the present study, we demonstrated that the GM700S, the OneTouch Verio IQ, the Contour NEXT and the Accu-Chek Aviva Plus fulfilled the 2013 ISO-like requirements [10]. In addition, we found that Accu-Chek Aviva Plus was the SMBG device with the highest usability among the six devices studied.
Previous studies have examined the accuracy of different SMBG systems according to the several ISO requirements over the years, but the reference method and the protocols used varied among them [12-17]. Brazg RL et al., examined the performance of 27 SMBG systems, including the Accu-Chek Aviva Plus in a population of 100 subjects [13]. This study was performed according to the ISO 15197:2003 requirements, but it was additionally tested for the ISO 15197:2013 criteria [13]. In the study by Brazg RL et al., perchloric acid hexokinase method was used as the reference method for measuring BG, while herein the YSI 2300 STAT PLUS was used [13]. The Accu-Check Aviva Plus fulfilled both ISO criteria and the percentages of the results within the required limits in all BG concentration were similar to the percentages found in our study.
Moreover, Bedini JL et al., evaluated the accuracy of Contour NEXT USB, OneTouch Verio IQ and FreeStyle InsuLinx® [14]. Tests were performed in 236 blood samples; unlike to our study, they used venous whole blood and not capillary blood and a hexokinase glucose analyser as the reference method. All glucometers used in that study fulfilled the ISO 15197:2013. Both Contour NEXT USB and OneTouch Verio IQ showed similar percentages of the results within the required limits, as in our study. Furthermore, Freckmann et al. tested the accuracy of Contour NEXT USB and OneTouch Verio IQ using the YSI 2300 STAT Plus as a reference method [17]. OneTouch Verio IQ failed to fulfil the ISO 15197:2013 ISO requirements, with only 84.6% of the results in BG levels <100 mg/dL and 91.9% of the results in BG levels ≥ 100 mg/dL within the required criteria. When tighter criteria were used (±10 mg/dL or ±10% and ±5 mg/dL or ±5%), the performance of OneTouch Verio IQ was worse than in our study, while similar to our findings the Contour NEXT USB had excellent performance in all BG range [17].
Regarding the GM700S there are not any previous studies that have examined the accuracy according to the ISO 15197:2013 requirements. However, Yu-Fei W et al., examined the performance of GM700, which is the previous model of GM700S; the reference method was the same, the YSI 2300 STAT PLUS [12]. GM700S satisfied the ISO 15197:2013 requirements. On the top of that, when GM700S was tested using tighter criteria (±10 mg/dL or ±10%) 100.0% of the results in BG levels <100mg/dL were within the required limits in both studies by Yu-Fei W et al., and our study.
Berti F et al., demonstrated that the Glucomen Areo satisfied the ISO 15197:2013 requirements [15]. The study by Berti F et al., was performed by A. Menarini Diagnostics, while the hexokinase-based method (Cobas Intergra 400 plus, Roche Instrument Center, Rotkreuz Switzerland) was used as reference method; in that study, more than 100 subjects participated and capillary blood samples were collected. In BG concentration <100mg/dL, 94.6 -100.0% of the results of the Glucomen Areo were within the required limits. However, only 91.3% of the results were within the required limits in our study. Nevertheless, in BG levels ≥100mg/dL similar percentages were found in the present study and in the study by Berti F et al., [15].
One previous study performed from the manufacturing company examined the accuracy of the FreeStyle Optium Neo device [16]. In that study, a total of 186 blood samples were collected from 165 patients and the YSI 2300 STAT PLUS was used as the reference method. FreeStyle Optium Neo fulfilled the ISO 15197:2013 criteria in that study, while we found that only 92.5% of the results in BG levels <100mg/dL and 85.0% of the results in BG concentrations ≥100mg/dL were within the required limits.
Although, most of the examined SMBG systems satisfied the ISO requirements, when tighter criteria were applied (±10 mg/dL or ±10%), the performance was strongly negatively affected. Hence, even though nowadays new systems, such as continuous glucose monitoring systems, are coming into the surface, the improvement of the classic SMBG systems should not be withheld, since they are still used by most people with diabetes mellitus. Consequently, they should be accurate and safe for use, especially when BG concentration is low and the threshold between hypoglycaemia and normoglycaemia is crucial.
A new finding of our study was patients’ preference about the usability of the SMBG systems. Physicians often neglect the fact that patients are concerned not only about the accuracy, but find usability and design equally important, especially younger people with type 1 DM who constantly carry their SMBG devices. Additionally, other aspects, such as the resolution of the SMBG device screen or the size of the numbers on the screen are very important especially for older people. Interestingly, although the FreeStyle Optium Neo had the worst performance of all six SMBG systems examined, it was favoured by the users due to the easiness on reading the numbers on the screen. The same applies for the Glucomen Areo that had inadequate performance, but was considered by the participants as one of the devices with the easiest blood sample application on the test strip. On the other hand, while the GM700S had the best performance in all BG levels according to both ISO 15197:2013 and to tighter criteria; it failed to gain patients preference. It is thus obvious that accuracy is not the only factor when choosing a SMBG device, but usability by the patients is also essential.
One of the strengths of our study is that the population of 120 participants is larger than the population (100 subjects) needed according to the ISO 15197:2013 requirements.
Limitation
One limitation of the present study is that we did not perform the protocol exactly as it is defined in the ISO 15197:2013 requirements; we used two strip lots instead of three strip lots. In addition, the use of YSI 2300 STAT PLUS as a reference method, which uses glucose oxidase, could be a possible limitation, since several SMBG systems are calibrated against a hexokinase method and a systematic difference (bias) is reported between hexokinase and glucose oxidase methods. Furthermore, the protocols of several referenced studies for the tested devices vary from this study and therefore, it is incorrect to directly compare the numerical percentages and draw conclusions. Finally, another possible limitation is that the accuracy of these SMBG systems was assessed when they were used by trained Health Care professionals and not by the patients themselves.
Conclusion
In conclusion, most of the examined glucometers which are commonly used in Greece had adequate performance when they were tested in criteria resembling to the ISO 15197:2013. Besides accuracy, usability of glucose meters is an important factor to be considered by the manufacturers of the devices.
CEG: Consensus Error Grid, SMBG: Self-Monitoring Blood Glucose, BG: Blood Glucose
SMBG: Self-Monitoring Blood Glucose
*Blood measurements were performed twice for each patient; SMBG: self-monitoring blood glucose, BG: blood glucose
SMBG: Self-Monitoring Blood Glucose
Manufacturer details for each SMBG studied: GM700S (Bionime, Berneck, Switzerland), OneTouch Verio IQ (LifeScan, Chesterbrook, PA, USA), Freestyle Optium Neo (Abbott Diabetes Care, North Chicago, IL, USA), Contour NEXT (Ascensia Diabetes Care, Parsippany, NJ, USA), Accu–Chek Aviva Plus (Roche Diabetes Care Inc., Indianapolis, IN, USA) and Glucomen Areo (A. Menarini Diagnostics, FIorence, Italy).