Introduction
Gender and obesity related influences on the experience of pain have received considerable empirical attention in recent years. Differences in pain sensitivity among individuals may have implications for pain management which might account in part for the variability in analgesic requirements between individuals.
Aim
To investigate the correlation of gender and serum leptin level with analgesic modulation of tramadol in high fat diet induced obese Wistar rats.
Materials and Methods
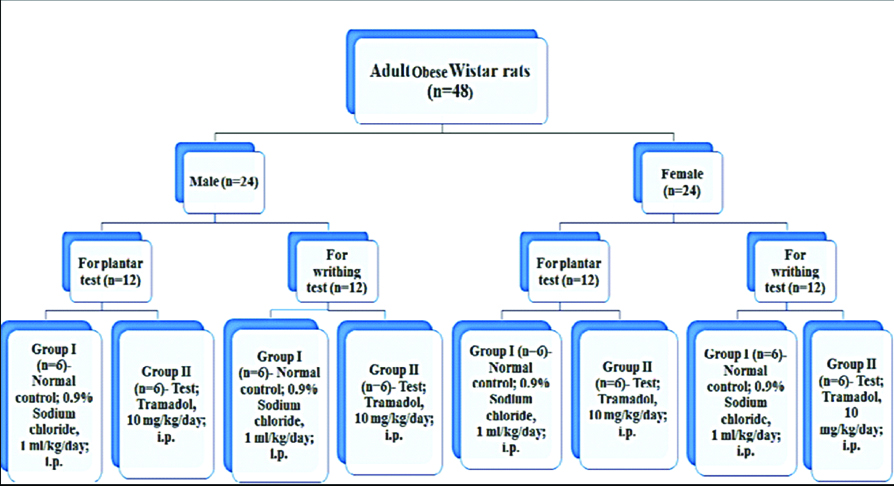
A total of 48 Wistar rats (24 each male and female; body weight 100-150 gm) were housed as two rats/cage. In addition to the normal pellet diet, these animals were orally fed with a mixture of Vanaspati daalda+Coconut oil (3:1)- 10 mL/kg/day for 90 days. After 90 days, these rats attained body weight approximately ≥300 gm (obese). Thereafter, each 24 male and 24 female obese rats were randomly divided into two groups (n=6/group) (Group I- Control: 0.9% NaCl; 1 mL/kg/day i.p. and Group II- Test: Tramadol 10 mg/kg/day i.p.) for each nociception model-plantar test (12 male rats and 12 female rats) and acetic acid induced writhing test (12 male rats and 12 female rats). The treatment duration was of five days.
Results
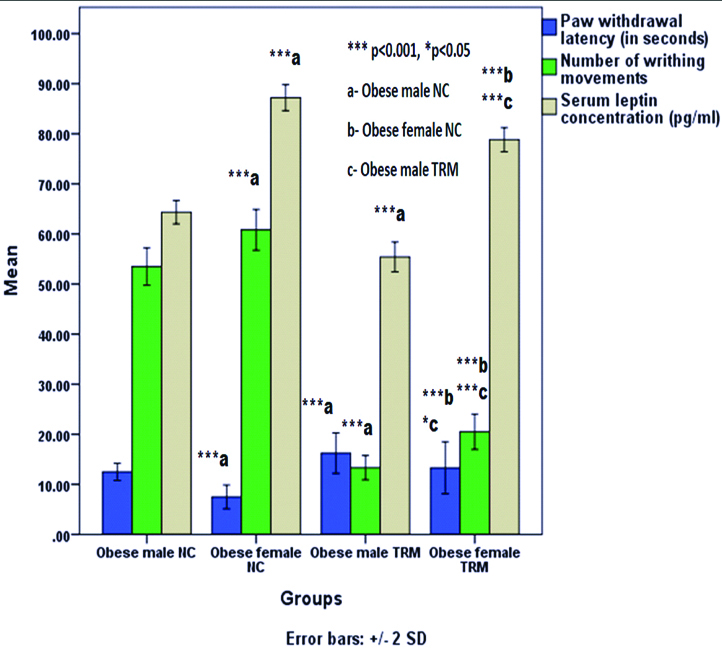
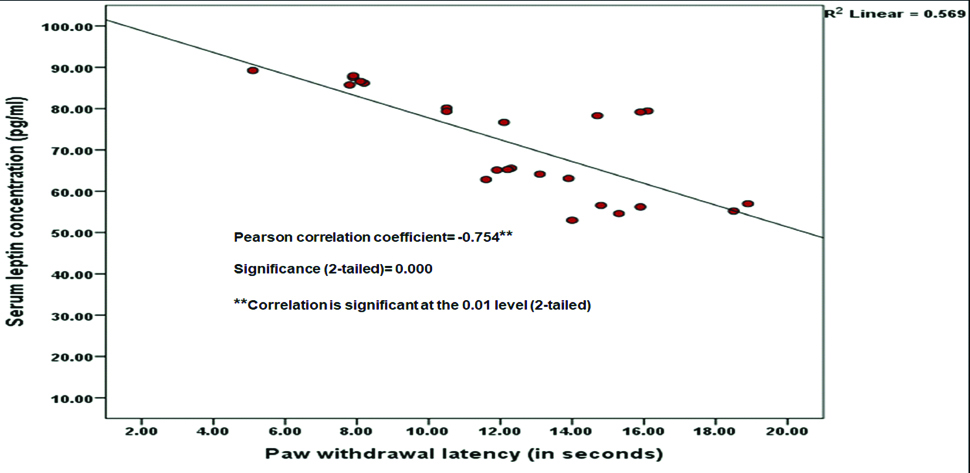
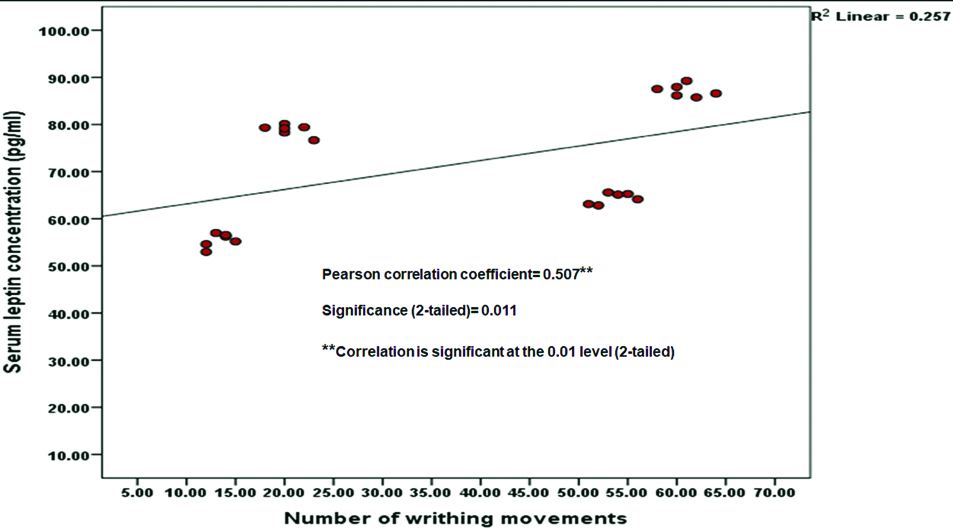
Paw Withdrawal Latency (PWL) was significantly decreased (p<0.001) and both number of writhing movements and serum leptin concentrations were significantly increased (p<0.001) in obese female control group compared to obese male control group. Tramadol treated both obese male and female rats had significantly increased PWL (p<0.001) and decreased both number of writhing movements and serum leptin concentration (p<0.001) in comparison with both obese male and female control groups respectively. In tramadol treated obese female rats, PWL was significantly decreased (p=0.048) and both number of writhing movements and serum leptin concentration were significantly increased (p<0.001) in comparison with the tramadol treated obese male rats. PWL was negatively correlated with serum leptin concentration (Pearson correlation coefficient= -0.754, 2-tailed significance; p<0.001) and number of writhing movements were positively correlated with serum leptin concentration (Pearson correlation coefficient=0.507, 2-tailed significance; p=0.011).
Conclusion
The present study revealed that obese female rats have more serum leptin concentration than obese male rats which could be one of the possible reasons for having more pain sensation to noxious stimuli in obese female rats compared to obese male rats. Tramadol treatment at the dose of 10 mg/kg for five days has decreased serum leptin level in rats which might be one of the additional analgesic mechanisms of action of tramadol.
Acetic acid, Obesity, Opioids, Pain threshold, Plantar, Sex hormone, Writhing test
Introduction
There is no one standard dose or specific medication that will provide optimum analgesia to all patients. Nowadays, the optimal current approach is to titrate analgesics to produce optimal therapeutic benefit. It is usually accepted that males and females respond differently to painful conditions. Gender related influences on experience of pain and efficacy of analgesics have received considerable empirical attention in recent years [1]. One of the meta-analysis suggests that females have more pain sensitivity for postoperative pain and less tolerance to pain compared to men [2]. Gender difference is not limited to pain perception but may extend to the response to analgesics. Gender difference in response to some of the non-opioid analgesics have also been reported [3]. There is little agreement about a differential response of men and women to opioid analgesics. In animals, male rats exhibited greater analgesia than female rats to equal doses of opioids [4,5]. In case of morphine, a gender difference is reported in its anti-nociceptive effect both in animals and human in spite of no gender-linked difference in serum levels of morphine [6-8]. In humans, sex differences in response to opioids have been described, however the findings are difficult to consolidate [6].
Several studies have reported the significant positive correlation for increasing pain sensitivity with an increase in Body Mass Index (BMI) [9,10]. Leptin is secreted by adipocytes in proportion to the amount of body fat and exerts a potent inhibitory action on food intake. In humans, serum leptin concentrations correlate positively with percent body fat. The causal relationship between the two remains unclear; it is not known whether obesity causes chronic pain, chronic pain causes obesity or some other factor causes both concurrently. Obesity is hypothesised to lead to pain because of excess mechanical stresses and its pro-inflammatory state due to more leptin. Obesity increases serum leptin levels due to more adipose tissues which can affect pain threshold, emotional mood and quality of life. One of the studies suggests that in obese patients, pain thresholds would make it possible to predict the need for prescriptions of drugs with a narrow therapeutic margin, such as morphine [11]. Mc Kendall MJ et al., conducted a study based on the hypothesis claiming that analgesic opiates are increased in obesity [12]. They applied a constant pressure of approximately three pounds to the tip of the thumb on 56 obese and non-obese patients to measure the time interval until the first sensation of pain. However, contrary to the expectations, the obese patients were found to be more sensitive to pain. Roane DS et al., found that Zucker rats are more sensitive to pain against tail flick pain stimulation tests [13].
Discrepancies in pain sensitivity and a decreased effect of analgesics among patients is decreasing their quality of life, increasing emotional and financial burden and further raising the healthcare cost of our country. There is need for further studies to investigate the correlation of gender and leptin with pain threshold and analgesic effect of opioids. Tramadol is a synthetic, centrally acting analgesic agent with two distinct, synergistic mechanisms of action, acting both as a weak opioid agonist and an inhibitor of monoamine neurotransmitter reuptake. Earlier, we had reported the analgesic modulation of some analgesics in male and female Wistar rats [14]. Thus, to establish an interrelationship among pain, opioids, gender and obesity is the focus of a growing body of research. Consequently, the aim of the present study was to investigate the correlation of gender and serum leptin level with analgesic modulation of tramadol in high fat diet induced obese Wistar rats.
Materials and Methods
This was a preclinical in vivo study. Following experimental protocol approval by the Institutional Animal Ethics Committee (IAEC/KMC/41/2014), this study was conducted for duration of about five months from March 2015-July 2015 at the premises of Central Animal Research Facility, Manipal Academy of Higher Education, Manipal, Karnataka, India.
Drugs and Reagents
Active Pharmaceutical Ingredient (API) of tramadol and rat leptin ELISA kit was procured from Sigma Aldrich, Bengaluru, India. Acetic acid and other chemicals were purchased from Merck Life Sciences Pvt. Ltd., Mumbai, India. Normal saline (0.9% Sodium chloride) was purchased from pharmacy of Kasturba Hospital, Manipal, Karnataka, India.
Animals
A total of 48 (24 male and 24 female) Wistar rats weighing 100-150 gm were housed in separate polypropylene cages, maintained under standard conditions with temperature (22-24°C), 12-h light/12-h dark cycle and relative air humidity 40-60%. The animals were acclimatised to the laboratory conditions for one week before the start of the experiment. The animals were provided with a normal pellet diet (Amrit Feeds Ltd., Pune, India) and water ad libitum. Experiments were conducted according to the ethical norms approved by Ministry of Social Justices and Empowerment, Government of India and Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA) guidelines.
Experimental Design
Diet induced obesity model: A total of 48 Wistar rats (24 each male and female; body weight 100-150 gm) were housed as two rats/cage. In addition to the normal pellet diet, these animals were orally fed mixture of Vanaspati daalda+Coconut oil (3:1)- 10 mL/kg/day for 90 days. After 90 days, these rats attained body weight approximately ≥300 gm (obese).
Thereafter, each 24 male and 24 female obese rats were randomly divided into two groups (n=6/group) (Group I- Control: administered 0.9% NaCl; 1 mL/kg/day i.p. and Group II- Test: administered tramadol 10 mg/kg/day i.p.) for each nociception model- plantar test (12 male rats and 12 female rats) and acetic acid induced writhing test (12 male rats and 12 female rats) [Table/Fig-1]. The treatment duration was of five days. On the last day of treatment (i.e., on 5th day); 15 minutes after tramadol/normal saline treatment, animals were tested for the respective pain model. PWL was assessed using plantar test and writhing movements were observed following administration of 0.8% acetic acid; 10 mL/kg i.p [14,15].
Nociception Models
Plantar test (Hargreaves’ method): Thermal pain threshold to radiant heat (IR- 90) was quantified using the paw withdrawal test. Rats were placed in a perspex enclosure, without restraint and a movable infrared radiant heat source placed directly under the plantar surface of the hind paw (Ugo Basile, Como, Italy). The PWL to radiant heat was defined as the time from onset of the radiant heat to the withdrawal of the rat hind paw. The cut-off time for PWL was 30 seconds. Testing was alternated between hind paws and carried out at three minutes intervals. The average of three estimations was taken to yield mean PWL. Before any testing was carried out, rats could adjust to their environments for at least 10 minutes [16].
Acetic acid induced writhing test: Writhing movement was induced by administering an intra-peritoneal injection of 0.8% acetic acid (10 mL/kg), 15 minutes after the tramadol/normal saline administration. After 10 minutes of acetic acid administration, the number of writhing movements such as abdominal constriction/elongation of body/arching of back/hind limb extension/forelimb extension/trunk twisting was cumulatively counted over 20 minutes further for nociceptive evaluation [14,15].
Collection of Blood Sample
Following anaesthesia with ketamine 60 mg/kg and xylazine 5 mg/kg; i.p., blood was withdrawn from retro-orbital plexus of rats through capillary tube [17]. Following collection of blood in microcentrifuge tubes and its clot formation, serum was obtained by centrifugation of blood at 3,000 rpm for 20 minutes at 4°C using a refrigerated centrifuge (MIKRO 22R, Andreas Hettich GmbH and Co. KG, Germany). The resulting supernatant (serum) was stored at -80°C for estimation of leptin concentration. All the surviving experimental animals were rehabilitated after the completion of study.
Estimation of Serum Leptin Concentration
As per the assay protocol given along with rat leptin ELISA kit, all reagents and samples were kept at room temperature (22-25°C) before use. One hundred microlitre (100 μL) of each standard and sample was added into appropriate wells. Wells were covered and incubated for 2.5 hours at room temperature or overnight at 4°C with gentle shaking. The solution was discarded and washed four times with 1X wash solution. Each well of microplate was washed with 300 μL wash buffer using a multichannel pipette. After the last wash, any remaining wash buffer was removed by aspirating or decanting. The plate was inverted and blotted against clean paper towels. One hundred microlitre (100 μL) of 1X prepared biotinylated detection antibody was added to each well followed by incubation for one hour at room temperature with gentle shaking. The solution was discarded. Washing was repeated four times. One hundred microlitre (100 μL) of prepared HRP-Streptavidin solution was added to each well followed by incubation for 45 minutes at room temperature with gentle shaking. Solution was discarded and washed again four times. One hundred microlitre (100 μL) of TMB reagent was added to each well followed by incubation for 30 minutes at room temperature in the dark with gentle shaking. Fifty microlitre (50 μL) of stop solution was added to each well and absorbance was read at 420 nm immediately. The mean absorbance for each set of duplicate standards, controls and samples were entered in the Microsoft Excel sheet and average zero standard optical density was subtracted from each mean absorbance. A standard curve with standard concentration on the x-axis and absorbance on the y-axis was plotted and following that leptin concentration (pg/mL) was calculated.
Statistical Analysis
Using Statistical Package for the Social Sciences (SPSS version 16.0; SPSS Inc., Chicago, USA), data were expressed as mean±standard deviation and analysed by One-way Analysis of Variance (ANOVA) followed by post-hoc Tukey test. The correlation of pain and serum leptin concentration was done by bivariate analysis followed by Pearson correlation coefficient and 2-tailed significance. A level for p≤0.05 was considered as statistically significant
Results
Paw withdrawal latency was significantly decreased (p<0.001) and both number of writhing movements and serum leptin concentrations were significantly increased (p<0.001) in female control group compared to male control group. Tramadol treated both male and female rats had significantly increased PWL (p<0.001) and decreased both number of writhing movements and serum leptin concentration (p<0.001) in comparison with both male and female control groups respectively. In tramadol treated female rats, PWL was significantly decreased (p=0.048) and both number of writhing movements and serum leptin concentrations were significantly increased (p<0.001) in comparison with the tramadol treated male rats [Table/Fig-2]. PWL was negatively correlated with serum leptin concentration (Pearson correlation coefficient=-0.754, 2-tailed significance; p<0.001), which means PWL was significantly decreasing (means more pain sensation to noxious stimuli) with increased levels of serum leptin [Table/Fig-3]. Number of writhing movements were positively correlated with serum leptin concentration (Pearson correlation coefficient= 0.507, 2-tailed significance; p=0.011), which means writhing movements were significantly increasing (means more pain sensation to noxious stimuli) with increased levels of serum leptin levels [Table/Fig-4].
Comparison of paw withdrawal latency (in seconds), number of writhing movements and serum leptin concentration (pg/mL) among obese rats.
n=6: number of rats in each group; NC: Normal control; TRM: Tramadol
Values are mentioned as mean. Error bars: +/- 2 standard deviation
Correlation between paw withdrawal latency (in seconds) and serum leptin concentration (pg/mL) in obese rats.
Correlation between number of writhing movements and serum leptin concentration (pg/mL) in obese rats.
Discussion
The present study has demonstrated the association of gender and leptin with analgesic modulation of tramadol in high fat diet induced obese Wistar rats. Leptin is secreted by adipocytes in proportion to the amount of body fat. In humans, serum leptin concentrations correlate positively with percent body fat. Several studies have positively correlated the experience of pain with an increase in BMI [9,10]. We found the significant increase in serum leptin levels and decrease in pain threshold (more pain sensation to noxious stimuli) in obese female rats than obese male rats. The significant increase in serum leptin concentration in obese female rats in the present study is in agreement with Mendonca HC et al., who found a positive and statistically significant correlation between oestrogen and leptin independent of body mass index [18]. According to Shimizu H et al., and Messinis IE et al., oestrogen can be an important regulator of leptin production in women [19,20]. The gender difference in serum leptin concentration is well established and in vitro results suggest that gonadal hormones, such as testosterone, may be important regulator of leptin secretion [21-23]. In both humans and rodents, males have lower plasma leptin concentrations than their female counterparts at any level of adiposity [24,25]. A strong inverse association between serum levels of leptin and testosterone was recently reported in untreated and testosterone-treated hypogonadal men [26,27]. In the present study, we also found significantly lower levels of leptin in male rats compared to female rats which might be due to down regulation of serum leptin by testosterone. The mechanism underlying this effect of testosterone remains to be elucidated.
The most studied of the endogenous pain modulatory systems is the endogenous opioid system and sex differences in the functioning of this system could arise based on several different mechanisms. First, sex differences could result from differences in the distribution, expression or sensitivity of opioid receptors in regions of the central nervous system involved in nociceptive processing. At rest, women have shown higher μ-opioid receptor binding in various cortical and subcortical brain regions than men [28], whereas men exhibited greater μ-opioid receptor binding in several brain regions than women in response to experimentally induced muscle pain. These sex differences in both resting and pain-related μ-opioid receptor binding may contribute not only to sex differences in basal pain perception but also to differences in sensitivity to opioid medications. Sex differences in opioid function could be partially mediated by the well-known interaction between gonadal hormones and the opioid system [29]. One of the studies suggests the incremental relationship between obesity and pain, that is, pain complaint became more prevalent as BMI status rose [30].
In the present study, tramadol treatment at the dose of 10 mg/kg for five days has decreased serum leptin level in both male and female obese rats. Some of the studies also suggest the decrease in serum leptin concentration with tramadol (opioid agonist) treatment at the dose of 0.5 mg/kg/day orally when administered for four weeks [30,31]. If we calculate the total amount of tramadol for five days in present study which comes to 50 mg/kg and in the above mentioned studies it is only 14 mg/kg in the total four weeks duration. Thus, the dose and duration of tramadol treatment in present study is even more than the above mentioned studies where serum leptin level was decreased. Decrease in serum leptin level by tramadol at the dose of 10 mg/kg for five days treatment duration could be one of the additional mechanisms of tramadol to reduce pain.
There was a significant positive correlation found between pain and serum leptin levels in present study. One of the effects of increased serum leptin levels may be greater sensitivity to pain. The relationship between leptin and pain may be modulated by other factors, with oestrogen being one possibility [32]. In rat and mouse models, leptin has been shown to reduce the threshold for pain [33]. Leptin has also been shown to increase levels of interleukin-1, a cytokine known to cause hyperalgesia [17]. Recent studies have shown that leptin, an adipocytokine, played a significant role in nociceptive behaviour induced by nerve injury in rats [34,35]. While the peripheral effect of leptin on neuropathic pain is mediated via macrophage stimulation [36]. Following more leptin levels in female rats compared to males, these could be the possible reasons for more pain in female rats than male rats.
Limitation
Usually there is species variation in pain and analgesic modulation of pain medications. Hence, the effect seen in animal studies cannot always be entirely extrapolated to humans. Additional research to elucidate the mechanisms driving sex hormones and leptin in pain sensitivity is needed in order to foster future interventions to reduce disparities in pain and analgesic modulation of tramadol. Gender and body mass index specific tailoring of pain treatments may become a conceivable outcome in the foreseeable future.
Conclusion
The present study concludes that obese female rats have experienced more pain sensation to noxious stimuli than obese male rats. Obese female rats also have more serum leptin concentration compared to obese male rats which could be one of the possible reasons for more pain sensation to noxious stimuli in obese female rats than male rats. Analgesic effect of tramadol is found to be more pronounced in obese male rats compared to obese female rats possibly due to impact of sex hormones. Tramadol treatment at the dose of 10 mg/kg for five days has decreased serum leptin level in rats which might be one of the additional mechanisms of tramadol to reduce pain.
[1]. Fillingim RB, Maixner W, Gender differences in the responses to noxious stimuliPain Forum 1995 4:209-11.10.1016/S1082-3174(11)80022-X [Google Scholar] [CrossRef]
[2]. Riley JL, Robinson ME, Wise EA, Myers CD, Fillingim RB, Sex differences in the perception of noxious experimental stimuli: A meta-analysisPain 1998 74:181-87.10.1016/S0304-3959(97)00199-1 [Google Scholar] [CrossRef]
[3]. Walkers JS, Carmody JJ, Experimental pain in healthy human subjects: Gender differences in nociception and in response to ibuprofenAnesth Analg 1998 86:1257-62.10.1213/00000539-199806000-00023 [Google Scholar] [CrossRef]
[4]. Kepler KL, Standifer KM, Paul D, Kest B, Pasternak GW, Bodnar RJ, Gender effects and central opioid analgesiaPain 1991 45:87-94.10.1016/0304-3959(91)90168-W [Google Scholar] [CrossRef]
[5]. Cicero TJ, Nock B, Meyer ER, Sex-related differences in morphine’s antinociceptive activity: relationship to serum and brain morphine concentrationsJ Pharmacol Exp Ther 1997 282:939-44. [Google Scholar]
[6]. Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD, Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesiaJ Pain 2005 6(2):116-24.10.1016/j.jpain.2004.11.00515694878 [Google Scholar] [CrossRef] [PubMed]
[7]. Cicero TJ, Nock B, Meyer ER, Gender-related differences in the anti-nociceptive properties of morphineJ Pharmacol Exp Ther 1996 279(2):767-73. [Google Scholar]
[8]. Kest B, Palmese C, Hopkins E, A comparison of morphine analgesic tolerance in male and female miceBrain Res 2000 879:17-22.10.1016/S0006-8993(00)02685-8 [Google Scholar] [CrossRef]
[9]. Finkelstein EA, Fiebelkorn IC, Wang G, State-level estimates of annual medical expenditures attributable to obesityObesity Research 2004 12(1):18-24.10.1038/oby.2004.414742838 [Google Scholar] [CrossRef] [PubMed]
[10]. Ayangco L, Sheridan PJ, World Health Organization. Obesity: Preventing and managing the global epidemic 1997 Geneva (Switzerland)World Health Organization [Google Scholar]
[11]. Lloret LC, Decleves X, Oppert JM, Basdevant A, Clement K, Bardin C, Pharmacology of morphine in obese patients: clinical implicationsClin Pharmacokinet 2009 48:635-51.10.2165/11317150-000000000-0000019743886 [Google Scholar] [CrossRef] [PubMed]
[12]. Mc Kendall MJ, Haier RJ, Pain sensitivity and obesityPsychiatry Research 1983 8:119-25.10.1016/0165-1781(83)90099-9 [Google Scholar] [CrossRef]
[13]. Roane DS, Porter JR, Nociception and opioid-induced analgesia in lean (Fa/-) and obese (fa/fa) Zucker ratsPhysiology Behaviour 1986 38:215-18.10.1016/0031-9384(86)90156-3 [Google Scholar] [CrossRef]
[14]. Chogtu B, Bairy KL, Satyam SM, Pirasanthan R, Gupta S, Analgesic Modulation of Tramadol, Amitriptyline and Gabapentin in Male and Female Wistar RatsResearch Journal of Pharmaceutical, Biological and Chemical Sciences 2013 4(3):70-78. [Google Scholar]
[15]. Motaghinejad M, Ebrahimzadeh A, Shabab B, Preventive effect of central administration of venlafaxine on morphine physical dependence, nociception, and blood cortisol level in ratInt J Prev Med 2014 5(11):1422-31. [Google Scholar]
[16]. Banik RK, Kabadi RA, A modified Hargreaves method for assessing threshold temperatures for heat nociceptionJ Neurosci Methods 2013 219(1):41-51.10.1016/j.jneumeth.2013.06.00523796910 [Google Scholar] [CrossRef] [PubMed]
[17]. Selim K, Sinan C, Suleyman S, Mete O, Mustafa S, Haluk K, Effects of central and peripheral administration of leptin on pain threshold in rats and miceNeuroendocrinology Lett 2003 24:193-96. [Google Scholar]
[18]. Mendonca HC, Montenegro RM, Foss MC, Silva de Sa MF, Ferriani RA, Positive correlation of serum leptin with estradiol levels in patients with polycystic ovary syndromeBraz J Med Biol Res 2004 37(5):729-36.10.1590/S0100-879X200400050001515107936 [Google Scholar] [CrossRef] [PubMed]
[19]. Shimizu H, Shimomura Y, Nakanishi Y, Futawatari T, Ohtani K, Sato N, Oestrogen increases in vivo leptin production in rats and human subjectsJournal of Endocrinology 1997 154:285-92.10.1677/joe.0.15402859291839 [Google Scholar] [CrossRef] [PubMed]
[20]. Messinis IE, Milingos S, Zikopoulos K, Kollios G, Seferiadis K, Lolis D, Leptin concentrations in the follicular phase of spontaneous cycles and cycles superovulated with follicle stimulating hormoneHuman Reproduction 1998 13:1152-56.10.1093/humrep/13.5.11529647537 [Google Scholar] [CrossRef] [PubMed]
[21]. Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Effects of gender, body composition, and menopause on plasma concentrations of leptinJ Clin Endocrinol Metab 1996 81:3424-27.10.1210/jcem.81.9.87841098784109 [Google Scholar] [CrossRef] [PubMed]
[22]. Hickey MS, Israel RG, Gardiner SN, Considine RV, McCammon MR, Tyndall GL, Gender differences in serum leptin levels in humansBiochem Mol Med 1996 59:01-06.10.1006/bmme.1996.0056 [Google Scholar] [CrossRef]
[23]. Kennedy A, Gettys TW, Watson P, Wallace P, Ganaway E, Pan Q, The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditureJ Clin Endocrinol Metab 1997 82:1293-300.10.1210/jcem.82.4.38599100610 [Google Scholar] [CrossRef] [PubMed]
[24]. Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS, Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin actionNat Med 1995 1:1311-14.10.1038/nm1295-13117489415 [Google Scholar] [CrossRef] [PubMed]
[25]. Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadjian R, Sexual dimorphism in plasma leptin concentrationJ Clin Endocrinol Metab 1997 82:579-84.10.1210/jc.82.2.5799024258 [Google Scholar] [CrossRef] [PubMed]
[26]. Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, Testosterone replacement increases fat-free mass and muscle size in hypogonadal menJ Clin Endocrinol Metab 1997 82:407-13.10.1210/jc.82.2.4079024227 [Google Scholar] [CrossRef] [PubMed]
[27]. Behre HM, Simoni M, Nieschlag E, Strong association between serum levels of leptin and testosterone in menClin Endocrinol (Oxf) 1997 47:237-40.10.1046/j.1365-2265.1997.2681067.x9302400 [Google Scholar] [CrossRef] [PubMed]
[28]. Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Muopioid receptor-mediated antinociceptive responses differ in men and womenJ Neurosci 2002 22:5100-107.10.1523/JNEUROSCI.22-12-05100.200212077205 [Google Scholar] [CrossRef] [PubMed]
[29]. Bodnar RJ, Commons K, Pfaff DW, Central neural states relating sex and pain 2002 BaltimoreJohns Hopkins University Press [Google Scholar]
[30]. Hitt HC, McMillen RC, Thornton-Neaves T, Koch K, Cosby AG, Comorbidity of obesity and pain in a general population: results from the Southern Pain Prevalence StudyJ Pain 2007 8(5):430-36.10.1016/j.jpain.2006.12.00317337251 [Google Scholar] [CrossRef] [PubMed]
[31]. Ibrahim IY, Ibrahim HM, Aziz NM, Rahman DMA, Effect of opioid receptors modulation on the HFD-Induced obesity in adult male Albino ratsMJMR 2014 25(2):72-81. [Google Scholar]
[32]. Anghel A, Jamieson C, Ren X, Young J, Porche R, Ozigbo E, Gene expression profiling following short-term and long-term morphine exposure in mice uncovers genes involved in food intakeNeurosci 2010 167:554-66.10.1016/j.neuroscience.2010.01.04320144693 [Google Scholar] [CrossRef] [PubMed]
[33]. Alvarez P, Bogen O, Chen X, Giudice LC, Levine JD, Ectopic endometrium-derived leptin produces oestrogen dependent chronic pain in a rat model of endometriosisNeuroscience 2014 258:111-20.10.1016/j.neuroscience.2013.11.00824239717 [Google Scholar] [CrossRef] [PubMed]
[34]. Hosoi T, Okuma Y, Nomura Y, Leptin regulates interleukin-1beta expression in the brain via the STAT3-independent mechanismsBrain Res Molec Brain Res 2002 13:139-46.10.1016/S0006-8993(02)02974-8 [Google Scholar] [CrossRef]
[35]. Lim G, Wang S, Zhang Y, Tian Y, Mao J, Spinal leptin contributes to the pathogenesis of neuropathic pain in rodentsJ Clin Invest 2009 119:295-304.10.1172/JCI36785PMC2631299 [Google Scholar] [CrossRef] [PubMed]
[36]. Maeda T, Kiguchi N, Kobayashi Y, Ikuta T, Ozaki M, Kishioka S, Leptin derived from adipocytes in injured peripheral nerves facilitates development of neuropathic pain via macrophage stimulationProc Natl Acad Sci USA 2009 106:13076-81.10.1073/pnas.090352410619620723 [Google Scholar] [CrossRef] [PubMed]