Eslicarbazepine Induced Stevens-Johnson Syndrome: A Rare Case Report
Kamal Nath1, Robin Victor2, Shruti Sharma3
1 Associate Professor, Department of Psychiatry, Silchar Medical College, Silchar, Assam, India.
2 Senior Resident, Department of Psychiatry, Himalayan Institute of Medical Sciences, Dehradun, Uttarakhand, India.
3 Postgraduate Trainee, Department of Psychiatry, Silchar Medical College, Silchar, Assam, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Robin Victor, Ladpur, Raipur Road, Dehradun-248001, Uttarakhand, India.
E-mail: robinvictor111@gmail.com
Stevens-Johnson Syndrome (SJS) is a rare severe cutaneous disease which may be fatal at times. One of the important aetiology of SJS is drug induced commonly due to drugs like carbamazepine, allopurinol, penicillin, sulfa drugs, ibuprofen, sodium valproate, phenytoin and lamotrigine etc. The objective of this article was to report a rare case of SJS/Toxic Epidermal Necrolysis (TEN) secondary to Eslicarbazepine administration in 28-year-old male who presented with chief complaints of fever, macular rashes over various body parts, difficulty in speaking and swallowing and painful ulceration of lips and tongue. The peculiarity about this case is that although cutaneous reactions have been reported in the past with carbamazepine group of drugs, the eruptions always occur within 4-5 days of drug administration when the drug has reached its steady state plasma concentration. Here the rashes started after two and a half weeks of starting the drug. Moreover, even on stopping Eslicarbazepine after admission to the hospital and initiation of treatment, new rashes continued to occur. Patient was managed with a combination of antihistamines, antibiotics, steroid and antipsychotics drugs to which he responded and was discharged after three weeks of admission and asked for regular follow-up. Hence healthcare professionals should be vigilant regarding the prescription of various drugs and prompt recognition and diagnosis of SJS for early initiation of treatment leading to a favourable outcome.
Adverse reaction, Anticonvulsant hypersensitivity syndrome, Drug induced, Maculopapular rash
Case Report
A 28-year-old Hindu male presented to the Emergency Department with complaint of fever with cough and vomiting since last three days, macular rashes over various body parts for two days, difficulty in speaking and swallowing for one day and painful ulceration of lips and tongue for one day prior to the day of admission.
History revealed that this patient was a diagnosed case of autistic spectrum disorder with mild intellectual disability who had visited many psychiatrists over the years for his various behavioural oddities as well as disorganised behaviour with impulsivity at times since childhood. Since last one year he also developed obsession of dirt and hygiene with compulsive behaviour. For this he was put on various psychotropic medications comprising of Tab. Risperidone (2 mg), Tab. Fluoxetine (20 mg), Tab. Olanzapine (5 mg). He was also suffering from hypothyroidism for which he was on Tab. Thyroxine (50 mcg) once daily. Patient was maintaining well on these medication and most of his symptoms were controlled. Around three weeks back, he developed excited behaviour with excessive talking which was mostly irrelevant with irritability and agitation on minor provocation and deterioration in personal hygiene following which, he was shown to another psychiatrist and prescribed Tab. Eslicarbazepine (400 mg), Tab. Quetiapine (50 mg) and Tab. Risperidone (1 mg) one of each daily at bedtime. Patient improved gradually over two and a half weeks when three days prior to admission he developed fever with diurnal variation which was accompanied with non-productive cough and multiple episodes of vomiting. Within the next day he developed macular lesions over various body parts which first appeared over the face mainly around the lips and angle of mouth and later progressed and involved the neck, shoulder, upper trunk and back. Next day i.e., one day prior to the day of admission patient developed difficulty in swallowing which was initially for the solid food and then for liquid food with pain and burning sensation of the throat while deglutition and development of ulcers on the lip and tongue.
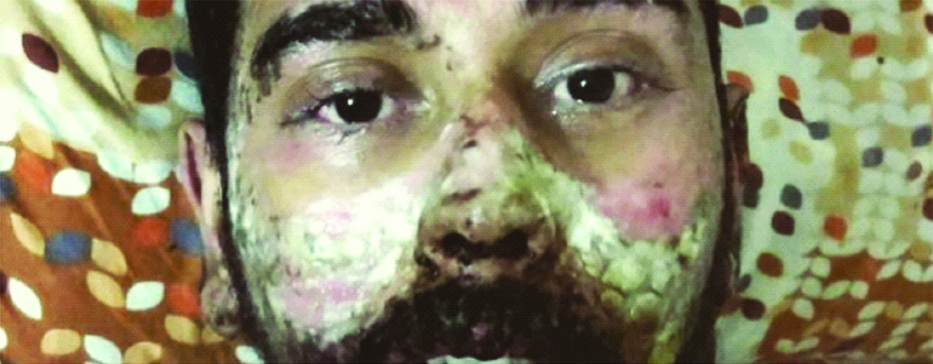
On the day of admission, the patient presented with swelling of both the eyes with reddening of conjunctiva accompanied by whitish discharge from eyes [Table/Fig-1].
Swelling of eyes with discharge.
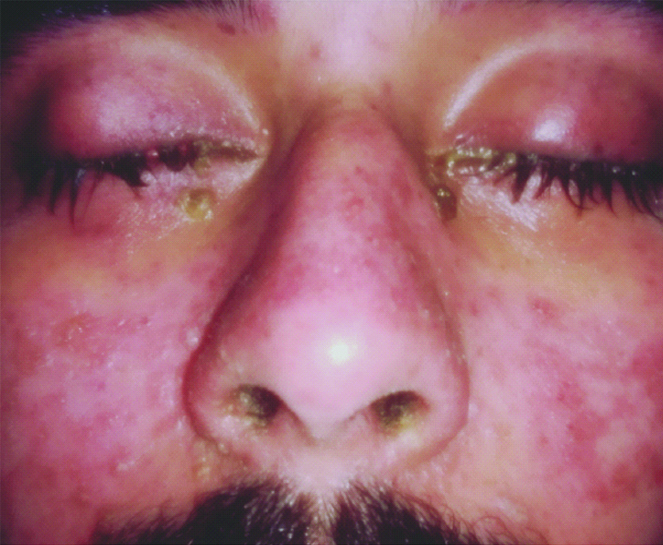
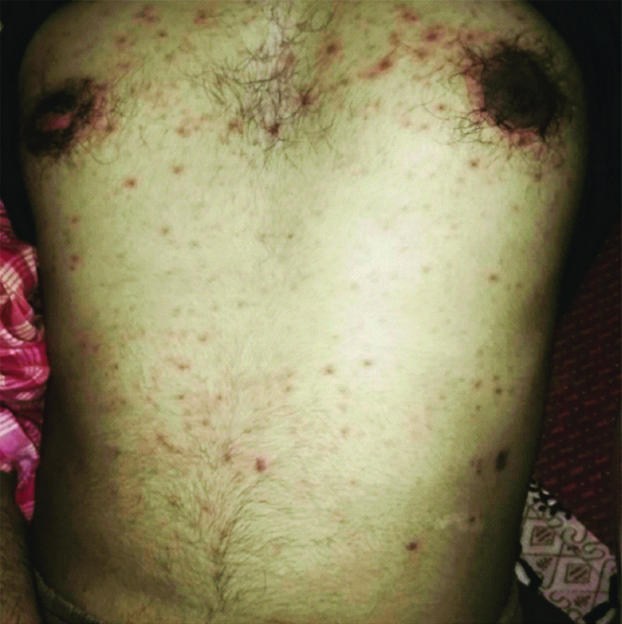
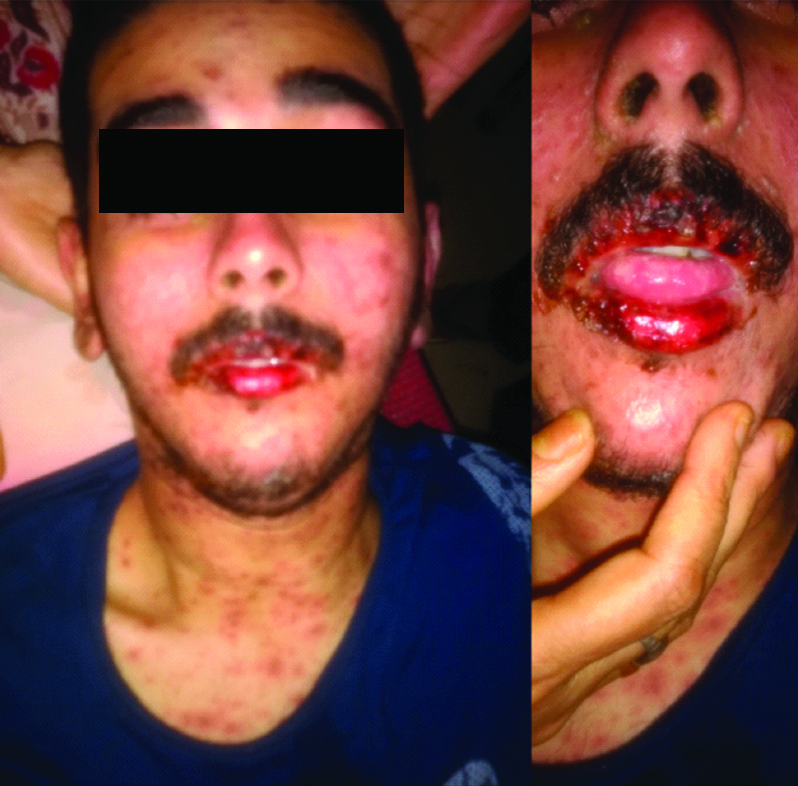
Most of the macular rashes were purpuric target like lesion and reddish in colour [Table/Fig-2]. There was also swelling and reddening of the lips of the patient with presence of multiple ulcers which were haemorrhagic on appearance and tender on palpation [Table/Fig-3].
Macular rashes and purpuric target like lesion.
Swelling and reddening of lips with ulceration of tongue.
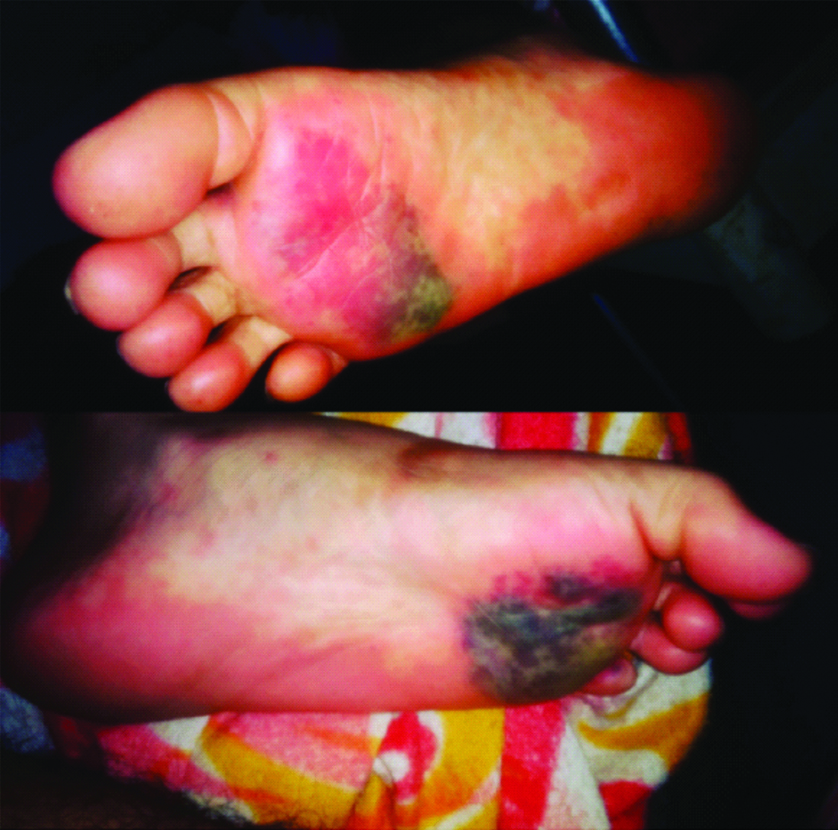
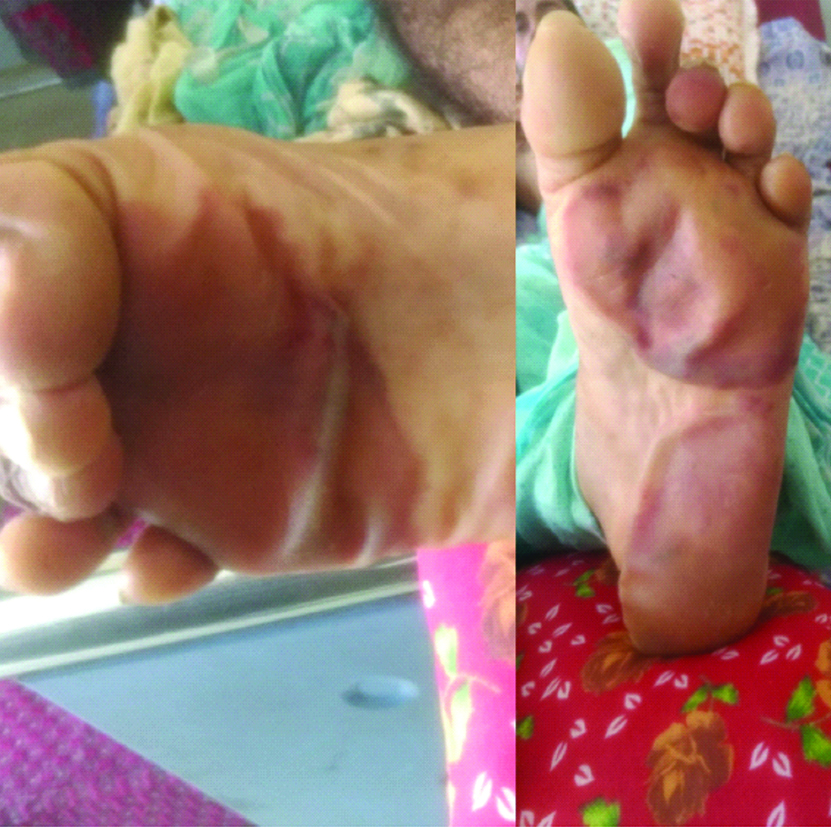
Based on history and examination, diagnosis of drug induced SJS was made. Patient was admitted under the Department of Dermatology and was started on Inj. Pheniramine Maleate IM twice a day, Inj. Cefotaxime+Sulbactam IV twice daily after negative skin test, Inj. Dexamethasone IV twice daily, Inj. Ondansetron IV twice daily, Inj. Pantoprazole IV once daily, Inj. Lorazepam IM stat and as and when required, Tab. Risperidone (1 mg) at bedtime, Tab. Quetiapine (25 mg) at bedtime, Moxifloxacin eye drops six times a day, artificial tear eye drops four times a day with intravenous fluids. Basic blood investigations were done and all haematological and biochemical parameters were found to be normal except mild hyponatremia (serum sodium 132 mEq/L). Within a day of admission, patient developed macular erythematous rashes over the lower limb mainly involving both the feet with blackish patches of necrosis measuring 1×4 cm over right sole of foot and 2×3 cm over the left sole of foot [Table/Fig-4].
Blackish necrotic patches over both soles.
Patient started responding to the medication in next 2-3 days and there was improvement of his appetite with healing of the rashes. After four days, most of the rashes over the face and trunk dried up and turned blackish with no new appearance of rashes [Table/Fig-5]. The swelling over the lips and ulceration over the tongue reduced. The swelling of the eyes and congestion of conjunctiva also subsided by the sixth day [Table/Fig-6] and the blackish necrotic lesion on both the soles of foot were filled with fluid which 2was then drained off with a syringe on the same day. The remaining fluid dried over a course of 1-2 weeks [Table/Fig-7]. After three weeks the patient was discharged with antibiotic ointment and eye drops. Since his behavioural symptoms had subsided by then, he was advised to review in psychiatry after two weeks.
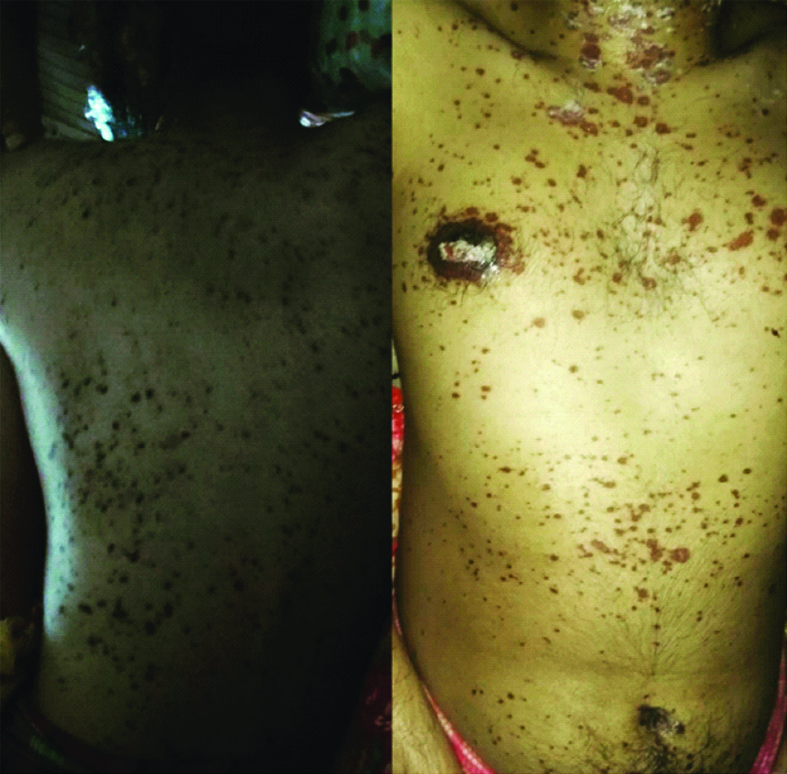
Dried rashes of face with improvement of eyes.
Dried necrotic patches over both the soles.
Discussion
Stevens-Johnson syndrome is a rare severe cutaneous disease which may be fatal at times. It is mainly drug induced, commonly due to drugs like carbamazepine, allopurinol, penicillin, sulfa drugs, ibuprofen, sodium valproate, phenytoin and lamotrigine etc. Other causes include herpes simplex virus or mycoplasma, vaccination, systemic diseases and certain food items. The reported incidence of SJS is around 2.6 to 6.1 cases per million people per year [1]. In 77-95% of the cases, drugs are implicated [2]. SJS is a part of a spectrum of disorders known as Severe Cutaneous Adverse Reactions (SCARs) which affects skin and mucous membranes. This spectrum includes erythema multiforme, SJS, TEN and SJS-TEN overlap and the classification into these types is based on the type of lesions and the extent of body surface area covered by the lesions [3].
The pathophysiology behind the syndrome is drug induced cytotoxic T lymphocytes causing widespread epithelial keratinocyte necrosis of both skin and mucous membrane. This causes clonal expansion of CD8+ cells due to Major Histocompatibility Complex Type I (MHC-I) restricted drug presentation. Also, soluble factors cause apoptosis of the keratinocytes. The death of these keratinocytes cause skin lesions mainly macules, small blisters and large blisters which may join together and which occur primarily on the torso [4]. These lesions cover 3-10% of body surface area in SJS, 10-30% in SJS-TEN overlap and more than 30% area in TEN. SJS is a serious systemic disorder which can lead to death at times. The mortality rate involved is very high, approximately 1-5% in SJS which may increase to 25-35% if more than 30% body surface area is involved [3]. Various research shows genetic association with the syndrome. It was seen that studies done in Taiwan on Han Chinese people pointed to a strong genetic predisposition to the development of carbamazepine-induced SJS/TEN in individuals bearing HLA-B*1502 [5]. Similar studies done in other Asian countries like Malaysia and Thailand showed the same risk of developing carbamazepine induced SJS in individuals with HLA-B*1502 [6].
Carbamazepine is antiepileptic drugs used to treat seizure disorder, bipolar disorders and neuropathic pain. It is also used as a second line mood stabiliser for the treatment and prevention of both phases of bipolar disorder [7]. It is known to produce a number of adverse effects like change in the production of red blood cells, white blood cells and platelets; increased risk of suicide, hyponatremia and Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH) and dangerous skin rashes [8]. The number of cases reported to cause carbamazepine hypersensitivity reaction is between 1/1,000 and 1/10,000 new exposures to the drug [1]. And among these reactions, SJS contribute a very small percentage. Eslicarbazepine being a newer drug in this carbamazepine group of drugs, very few reports are available in the existing literature about the Eslicarbazepine induced SJS [9]. To the best of our knowledge there are no such reports of Eslicarbazepine induced SJS from the Indian subcontinent.
The objective of this study was to report this rare case of SJS/TEN secondary to Eslicarbazepine administration.
Eslicarbazepine was approved by US Food and Drug Administration (FDA) in 2013 as an adjunctive therapy in adults with refractory partial onset seizure with or without secondary generalisation [10]. Nath K et al., reported that Eslicarbazepine could be used in successful management of refractory bipolar disorder [11]. Incidence of rash reported on Eslicarbazepine administration, although very rare were higher for doses more than 1200 mg/day as compared to 800 mg/day, however, it was not higher among patient initiated at 800 mg/day vs. 400 mg/day [10]. Its biological half-life is 10-20 hours and it reaches steady state plasma concentration within 4-5 days [7].
The patient was a case of autism spectrum disorder with mild intellectual disability and hypothyroidism since childhood who was on various psychotropic medications and prescribed Tab. Eslicarbazepine (400 mg), which is a newer carbamazepine for his affective symptoms. The peculiarity about this case is that although cutaneous reactions have been reported in the past with carbamazepine group of drugs, the eruptions mostly occur within 8-16 days of drug administration [12] when the drug has reached its steady state plasma concentration. However in this case, the rashes started after two and a half weeks of starting the drug. Moreover, even on stopping Eslicarbazepine after admission to the hospital, new rashes continued to occur. As already mentioned, there have been reports of increased risk of SJS in people with HLA-B*1502 however HLA typing could not be done in the patient due to lack of resources.
Although, there are many case reports of carbamazepine [13-15] and oxcarbazepine [16] induced SJS, very few reports [9] are available in the existing literature about the Eslicarbazepine induced SJS. Thus, one should be cautious while prescribing Eslicarbazepine and it should be started at a low dose and then titrated up slowly after explaining all the warning signs to the caregivers.
Conclusion
Healthcare professionals should be vigilant regarding the prescription of various drugs and prompt recognition and diagnosis of SJS well as other adverse reactions for early initiation of treatment leading to a favourable outcome.
[1]. Tennis P, Stern RS, Risk of serious cutaneous disorders after initiation of use of phenytoin, carbamazepine, or sodium valproate: a record linkage studyNeurology 1997 49(2):542-46.10.1212/WNL.49.2.5429270593 [Google Scholar] [CrossRef] [PubMed]
[2]. Roujeau JC, Stern RS, Severe adverse cutaneous reactions to drugsN Engl J Med. [Internet] 1994 331(19):1272-85.[cited 2017 Nov 21] Available from:http://www.ncbi.nlm.nih.gov/pubmed/779431010.1056/NEJM1994111033119067794310 [Google Scholar] [CrossRef] [PubMed]
[3]. Harr T, French LE, Toxic epidermal necrolysis and Stevens-Johnson syndromeOrphanet J Rare Dis. [Internet]. BioMed Central 2010 5:39[cited 2017 Jun 18] Available from:http://www.ncbi.nlm.nih.gov/pubmed/211627210.1186/1750-1172-5-3921162721 [Google Scholar] [CrossRef] [PubMed]
[4]. Hazin R, Ibrahimi OA, Hazin MI, Kimyai-Asadi A, Stevens-Johnson syndrome: pathogenesis, diagnosis, and managementAnn Med 2008 40(2):129-38.10.1080/0785389070175366418293143 [Google Scholar] [CrossRef] [PubMed]
[5]. Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci USA. [Internet]National Academy of Sciences 2005 102(11):4134-39.[cited 2017 Jun 18] Available from: http://www.ncbi.nlm.nih.gov/pubmed/1574391710.1073/pnas.040950010215743917 [Google Scholar] [CrossRef] [PubMed]
[6]. Nguyen D Van, Chu HC, Nguyen D Van, Phan MH, Craig T, Baumgart K, HLA-B*1502 and carbamazepine-induced severe cutaneous adverse drug reactions in Vietnamese. Asia Pac Allergy. [Internet]Asia Pacific Association of Allergy, Asthma and Clinical Immunology 2015 5(2):68-77.[cited 2017 Aug 21] Available from:http://www.ncbi.nlm.nih.gov/pubmed/2593807110.5415/apallergy.2015.5.2.6825938071 [Google Scholar] [CrossRef] [PubMed]
[7]. Editor JS, Editor V a S, Kaplan & Sadock’s Comprehensive Textbook of PsychiatryPsychiatry Interpers Biol Process. [Internet] 2000 II(1):4884Available from:http://www.amazon.co.uk/Sadocks-Comprehensive-Textbook-Psychiatry-Saddocks/dp/0781768993 [Google Scholar]
[8]. Durelli L, Massazza U, Cavallo R, Carbamazepine toxicity and poisoning. Incidence, clinical features and managementMed Toxicol Adverse Drug Exp [Internet] 1999 4(2):95-107.[cited 2017 Nov 21] Available from:http://www.ncbi.nlm.nih.gov/pubmed/265454510.1007/BF032599062654545 [Google Scholar] [CrossRef] [PubMed]
[9]. Alcántara-Reifs CM, Salido-Vallejo R, Garnacho-Saucedo G, de la Corte-Sánchez S, Vélez García-Nieto A, Eslicarbazepine-induced toxic epidermal necrolysisEpilepsia [Internet] 2016 57(5):854-55.[cited 2018 Feb 17] Available from: http://www.ncbi.nlm.nih.gov/pubmed/2716080110.1111/epi.1336327160801 [Google Scholar] [CrossRef] [PubMed]
[10]. Rocamora R, A review of the efficacy and safety of eslicarbazepine acetate in the management of partial-onset seizuresTher Adv Neurol Disord. [Internet] 2015 8(4)SAGE Publications:178-86.[cited 2017 Jun 19] Available from:http://www.ncbi.nlm.nih.gov/pubmed/2613684510.1177/175628561558971126136845 [Google Scholar] [CrossRef] [PubMed]
[11]. Nath K, Bhattacharya A, Praharaj SK, Eslicarbazepine acetate in the management of refractory bipolar disorderClin Neuropharmacol [Internet] 2012 35(6):295-29.[cited 2017 Jun 19] Available from:http://www.ncbi.nlm.nih.gov/pubmed/2315146910.1097/WNF.0b013e318271220b23151469 [Google Scholar] [CrossRef] [PubMed]
[12]. Mehta M, Shah J, Khakhkhar T, Shah R, Hemavathi KG, Anticonvulsant hypersensitivity syndrome associated with carbamazepine administration: Case seriesJ Pharmacol Pharmacother [Internet] 2014 5(1)Wolters Kluwer-Medknow Publications:59-62.[cited 2018 Feb 17] Available from:http://www.ncbi.nlm.nih.gov/pubmed/2455491410.4103/0976-500X.12442824554914 [Google Scholar] [CrossRef] [PubMed]
[13]. Khoo ABS, Ali FR, Yiu ZZN, Ferguson JE, Carbamazepine induced Stevens-Johnson syndromeBMJ Case Rep [Internet] 2016 2016(1)BMJ Publishing Group Ltd:bcr2016214926[cited 2017 Jun 19] Available from: http://www.ncbi.nlm.nih.gov/pubmed/2696936810.1136/bcr-2016-21492626969368 [Google Scholar] [CrossRef] [PubMed]
[14]. Avinash A, Amberkar VM, Kunder SK, Madhyastha S, Meenakumari K, Carbamazepine-induced Life-threatening Stevens-Johnson Syndrome and Agranulocytosis: The Maiden CaseJ Clin Diagn Res [Internet] 2016 10(12)JCDR Research& Publications Private Limited:FD01-FD03.[cited 2017 Jun 19] Available from:http://www.ncbi.nlm.nih.gov/pubmed/28208879 [Google Scholar]
[15]. Bae HM, Park YJ, Kim YH, Moon DE, Stevens-johnson syndrome induced by carbamazepine treatment in a patient who previously had carbamazepine induced pruritus-a case reportKorean J Pain [Internet] 2013 26(1)Korean Pain Society:80-83.[cited 2017 Jun 19] Available from:http://www.ncbi.nlm.nih.gov/pubmed/2334221410.3344/kjp.2013.26.1.8023342214 [Google Scholar] [CrossRef] [PubMed]
[16]. Sharma SR, Sharma N, Yeolekar ME, Oxcarbazepine-induced Stevens Johnson syndrome: A rare case reportIndian Dermatol Online J [Internet] 2011 2(1)Medknow Publications:13-15.[cited 2017 Jun 19] Available from: http://www.ncbi.nlm.nih.gov/pubmed/2313020710.4103/2229-5178.7986123130207 [Google Scholar] [CrossRef] [PubMed]