According to the world health organisation, 41 million children under the age of five are known to be obese worldwide [1]. Children who are identified as obese during childhood are at a higher risk of developing complications related to metabolic syndrome in adulthood [2]. With increasing prevalence of childhood obesity, it is important to understand the underlying aetiologies associated with it. Foetal growth and development is determined by a combination of genetic and environmental factors. During pregnancy, growth of the foetus is highly influenced by the in utero environment. Therefore, maternal nutrition plays a major role not only on the mother’s own health however, it has a lasting impact on the normal growth and the well-being of the baby [3]. There are increasing evidence to suggest that infants who are born LGA are at a higher risk of developing obesity and metabolic syndrome later in life [4,5]. Although, foetal macrosomia is more prevalent in mothers with gestational diabetes, the rate of newborns with macrosomia born to non-diabetic mothers is also increasing worldwide [6]. Hence, foetal growth is affected by maternal genetic, demographic and metabolic factors [7]. Maternal demographic variables such as pre-pregnancy BMI, gestational weight gain, parity, gestational age at delivery independently predict the birth weight of the newborn [8]. Maternal lipid metabolism is also important during foetal development. During a normal pregnancy, as the gestation age progresses, an increase in maternal total cholesterol and triglyceride levels in serum is observed [9]. Both triglyceride and total cholesterol are transported to the foetus after they are being absorbed by the placenta and being metabolised there. However, high levels of maternal triglycerides and total cholesterol levels are associated with pregnancy induced hypertension, pre-eclampsia, pre-term birth and LGA [10-13]. On the other hand, low levels of serum triglyceride levels and total cholesterol in the mother are related to pre-term birth and birth of SGA babies [10,14].
Epidemiologic studies done on the Asian population revealed that Asians have a higher risk of Type 2 diabetes mellitus and cardiovascular disease compared to populations of European descent for any given BMI [15]. They have also shown that the Asians have a higher risk for components of metabolic syndrome such as B-cell dysfunction, insulin resistance, dyslipidaemia and hepatic steatosis at much lower waist circumferences and BMIs [16]. It is already proven that South Asians have a higher body fat content for any given BMI [17-19]. Recent studies also revealed that adipose tissue metabolism in Asians is different compared to that of other populations and lower levels of adiponectin, which has a positive correlation with metabolic syndrome, was observed in them [20]. In this study, it is hypothesised that the increased serum levels of lipid in pregnant mothers during late pregnancy has a higher correlation with the birth of LGA babies in Southern Sri Lanka. Therefore, the objectives of the present study are to assess the prevalence of birth of LGA babies, to determine the associations between aetiologies and the birth of LGA babies in a tertiary care setting in Southern Sri Lanka, and to determine whether changes in maternal lipid profile are associated with the birth of LGA babies.
Materials and Methods
This study was conducted at the professorial unit of the Teaching Hospital, Mahamodara, Galle, Sri Lanka. It is a Tertiary Care Centre attached to the Department of Obstetrics and Gynaecology, Faculty of Medicine, University of Ruhuna, Galle, Sri Lanka. Ethical clearance was obtained from the Ethical Review Committee, Faculty of Medicine, University of Ruhuna, Galle, Sri Lanka.
A case-control study was conducted which consisted of two arms. In the first arm of the study, 149 postnatal mothers aged 18-35 years and their newborns were recruited by convenience sampling [Table/Fig-1]. The age limit 18-35 years was chosen as it represents a population of pregnant women with a relatively low risk. The study sample consist of Sinhala, Tamil, Muslim pregnant mothers which includes all ethnic groups in Sri Lanka. A written consent for the participation was obtained from each participant by giving an information sheet after introducing them the project and the objectives of the study. Data were collected using an interviewer administered, self-developed questionnaire which was cross checked with the antenatal records of the mother. All mothers who delivered appropriate and LGA babies during a period of one month (December 2016 to January 2017) were included in the study and the mothers or newborns who had complications during the delivery, SGA babies or who refused to participate in the study were excluded. All mothers were interviewed after the delivery to obtain information on maternal age, monthly income, educational status, occupation, pregestational BMI, gestational weight gain, number of previously born children and also on complications associated with birth of LGA babies.
Flow chart describing the procedure used in the study.
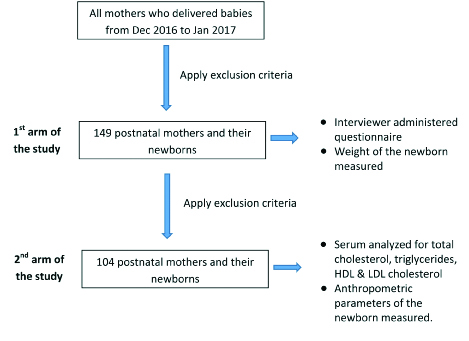
In the second arm of the study, 104 postnatal mothers aged 18-35 years and their newborns were recruited by convenience sampling [Table/Fig-1]. These pregnant mothers and newborns overlapped with the study sample which was included in the first arm of the study but the sample number was reduced due to application of extra exclusion criteria. Women who gave birth to twins, delivered pre term babies i.e., with period of gestation <37 weeks, had diabetes (pre-existent as well as gestational diabetes), and had congenital abnormalities were excluded. Those who were on lipid-altering medication such as antiepileptic drugs, steroids, insulin, antidepressents, and medications for thyroid disorders or sleep deprivation were also excluded. From the pregnant mothers who were admitted to the professorial unit for the delivery, 3 mL of venous blood was collected into a plain tube after a 10 hour fast. Serum was separated and stored at -20°C until analysis. Every sample was assayed for total cholesterol, triglycerides and HDL-cholesterol concentration and LDL cholesterol concentration was calculated by the Friedewald equation. Total cholesterol concentration was assayed with the cholesterol oxidase-phenol aminophenazone method and triglyceride concentration was assayed using the glycerol-3 phosphatase oxidase phenol aminophenazone method. HDL-cholesterol was measured by homogeneous enzymatic colorimetric assays. Anthropometric parameters of newborns {BW (to the nearest 0.1 kg), length of the newborns (to the nearest 0.1 cm) and head circumferences} were measured with the help of a well-trained nursing officer in the labour room. Birth weights and gestational age of all the newborns were collected from relevant labour rooms. The gestational age was estimated by last menstrual period, confirmed by ultrasonography before 20 weeks of gestation. According to Centre for Disease Control (CDC) and Prevention guidelines, infants with birth weights >90th percentile for gestational age and sex were classified as LGA and those having weight between 10th and 90th percentile were classified as Appropriate for Gestational Age (AGA).
Statistical Analysis
Statistical analysis was performed using Statistical Package for Social Sciences (SPSS) 16.0 for Windows software. Data were expressed as Mean±Standard Deviation (SD), number (percentages) or frequency. Differences in categorical variables were tested using a chi-square test. To find out the associations among total cholesterol, triglycerides, HDL cholesterol and LDL cholesterol, descriptive analysis was done to explain the variations in the variables used. Logistic regression and correlation coefficient were used to determine the relationships between variables. (Statistical difference was defined as p<0.05).
Results
[Table/Fig-2] shows the prevalence of LGA infants at the Teaching Hospital, Mahamodara, Sri Lanka. According to the CDC guidelines, out of 834 newborns born within a period of one month, birth weight of 103 newborns (12.35%) exceeded the 90th percentile and 39 newborns (4.67%) exceeded the 97th percentile.
Prevalence of large for gestational age infants at the Teaching Hospital, Mahamodara, Sri Lanka.
| Definition | Prevalence |
|---|
| >90th percentile | 12.35% |
| >97th percentile | 4.67% |
Maternal demographic characteristics are shown in [Table/Fig-3,4] There were no association between the birth of LGA infants and the maternal age, monthly income, educational state, occupation of the mother, pre gestational BMI and gestational weight gain. Out of all mothers participated in the study, 69.8% had a gestational weight gain lower than the recommended level. Therefore, gestational weight gain was not a significant risk factor to be LGA. Period of gestation showed a significant association for the birth of LGA babies. However, majority of mothers were in the normal range of 37-40 weeks of gestation. Sex of the newborn also had a significant association with the birth of LGA babies where more males were born than females. Although, a significant association was shown between the mode of delivery and the birth of LGA babies, it was towards the normal vaginal delivery.
Maternal demographic characteristics.
| Demographic characteristics | LGA | AGA | p-value |
|---|
| Maternal age |
|---|
| 18-20 | 1 (8.3%) | 11 (91.7%) | 0.153 |
| 21-30 | 19 (22.4%) | 66 (77.6%) |
| >30 | 17 (32.7%) | 35 (67.3%) |
| Monthly income (Sri Lankan rupees) |
|---|
| 10,000-30,000 | 20 (27%) | 54 (73%) | 0.708 |
| 30,001-50,000 | 6 (19.4%) | 25 (80.6%) |
| >50,000 | 11 (25%) | 33 (75%) |
| Educational status of the mother |
|---|
| Primary | 1 (25%) | 3 (75%) | 0.981 |
| Secondary | 33 (24.6%) | 101 (75.4%) |
| Tertiary | 3 (27.3%) | 8 (72.7%) |
| Occupation of the mother |
|---|
| Skilled worker | 5 (33.3%) | 10 (66.7%) | 0.784 |
| Professional worker | 2 (25%) | 6 (75%) |
| Housewife | 30 (23.8%) | 96 (76.2%) |
| Ethnicity of the mother |
|---|
| Buddhists | 29 (22.3%) | 101 (77.7%) | 0.174 |
| Muslims | 6 (42.9%) | 8 (57.1 %) |
| Others | 2 (40%) | 3 (60%) |
Data were collected in the 1st arm of the study (n=149). Data were expressed as n (%). Statistical analysis between large for gestational age (LGA) and appropriate for gestational age (AGA) groups were performed by the chi-square test while maternal age, pre-gestational body mass index (BMI), period of gestation were analysed by Kruskal Wallis test. AGA: Appropriate for gestational age, LGA: Large for gestational age, BMI: Body Mass Index. *p<0.05 was considered significant.
Maternal characteristics related to the pregnancy.
| Variables | LGA | AGA | p-value |
|---|
| Maternal characteristics |
|---|
| Pre-gestational BMI of the mother |
|---|
| <18.4 | 8 (27.6%) | 21 (72.4%) | 0.909 |
| 18.4-24.9 | 21 (24.7%) | 64 (75.3%) |
| >25.0 | 8 (22.9%) | 27 (77.1%) |
| Gestational weight gain |
|---|
| Low weight gain | 23 (22.1%) | 81 (77.9%) | 0.331 |
| Normal weight gain | 12 (34.3%) | 23 (65.7%) |
| High weight gain | 2 (20 %) | 8 (80%) |
| Number of previously born children |
|---|
| None | 11 (18%) | 50 (82%) | 0.210 |
| One | 17 (34.7%) | 32 (65.3%) |
| Two | 7 (21.2%) | 26 (78.8%) |
| >2 | 2 (33.3%) | 4 (66.7%) |
| Presence of gestational diabetes |
|---|
| Presence | 1 (16.7%) | 5 (83.3%) | 0.637 |
| Absence | 36 (25.2%) | 107 (74.8%) |
| Period of gestation |
|---|
| <36 months | 4 (10.8%) | 0.00 (0.00%) | 0.001* |
| 36-40 months | 28 (75.6%) | 82 (73.2%) |
| >40 months | 5 (13.5%) | 30 (26.8%) |
| Sex of the last child |
|---|
| Male | 14 (19.4%) | 58 (80.6%) | 0.161 |
| Female | 23 (29.9%) | 54 (70.1%) |
| Year gap between last child and newborn baby |
|---|
| None | 11 (18%) | 50 (82%) | 0.557 |
| 1-2 years | 3 (33.3%) | 6 (66.7%) |
| 2-3 years | 9 (34.6%) | 17 (65.4%) |
| >3 years | 14 (26.4%) | 39 (73.6%) |
| Sex of the newborn |
|---|
| Male | 25 (31.6%) | 54 (68.4%) | 0.041* |
| Female | 12 (17.1%) | 58 (82.9%) |
| Mode of delivery |
|---|
| Normal vaginal delivery | 22 (19.3%) | 88 (80.7%) | 0.016* |
| Cesarean delivery | 15 (38.5%) | 24 (61.5%) |
Data was collected in the 1st arm of the study (n=149). Data were expressed as n (%). Statistical analysis between large for gestational age (LGA) and appropriate for gestational age (AGA) groups were performed by the Chi-squared test while maternal age, pre-gestational Body Mass Index (BMI), period of gestation were analysed by Kruskal Wallis test. *p<0.05 was considered significant.
[Table/Fig-5] shows maternal and neonatal characteristics with respect to the association between maternal lipid profile and the birth of a LGA infants in a tertiary care setting in Sri Lanka. There were significantly higher levels (p<0.001) of serum triglycerides and significantly lower levels (p<0.001) of serum HDL in mothers who delivered LGA babies compared to mothers who delivered appropriate for gestational age babies. Consistently, anthropometric parameters of the newborns, birth weight, birth length and the head circumference were significantly higher in LGA infants. A significant change was not observed in the serum cholesterol concentration and the LDL concentration between the LGA and the AGA groups.
Comparison of lipid profile between large for gestational age (LGA) and appropriate for gestational age (AGA) newborns: 2nd arm of the study.
| Variables | Total | LGA | AGA | p-value |
|---|
| Total Cholesterol | 226.250 (204.000-259.220) | 230.650 (210.650-259.225) | 221.450 (200.325-259.650) | 0.416 |
| Triglycerides | 203.000 (172.950-250.9509) | 258.050 (226.475-298.950) | 186.000 (158.700-203.075) | <0.001* |
| HDL Cholesterol | 42.950 (38.600-53.500) | 38.000 (35.450-40.775) | 51.750 (43.475-57.5700 | <0.001* |
| LDL Cholesterol | 141.920 (117.155-172.750) | 143.670 (122.805-160.000) | 140.170 (109.650-177.090) | 0.752 |
| Birth weight (g) | 3250.000 (2950.000-3537.50) | 3600.000 (3400.000-3875.000) | 3050.000 (2750.000-3250.000) | <0.001* |
| Birth length (cm) | 54.000 (52.000-56.000) | 56.000 (54.000-57.000) | 52.500 (51.000-54.000) | <0.001* |
| Head Circumference (cm) | 34.000 (32.000-35.000) | 35.000 (33.000-36.000) | 33.000 (32.000-35.000) | 0.002* |
Data was collected in the 2nd arm of the study (n=104). Data were presented as median (interquartile range). Statistical analysis was performed by the Kruskal Wallis test for the differences between the large for gestational age (LGA) and appropriate for gestational age (AGA) groups. *p<0.001.
There was an intermediate correlation (r=0.529) between serum triglyceride levels and the birth weight of the newborn [Table/Fig-6]. When maternal serum triglyceride level increases, birth weight of the newborn also increased. There was also a correlation between maternal serum triglyceride level and the length of the newborn (r=0.485) and the head circumference (r=0.228) of the newborn. When maternal serum triglyceride level increases, both the length and the head circumference of the newborns increase.
Correlation among anthropometric parameters of newborns born appropriate and large for gestational age and lipid profile of the birth mothers
| Anthropometric parameters | POA | Birthweight | Length | Head circumference | Total cholesterol | Triglycerides | HDL | LDL |
|---|
| POA | 1 104
| 0.243* 0.013 104
| 0.104 0.294 104
| 0.146 0.139 104
| -0.137 0.166 104
| -0.106 0.282 104
| 0.152 0.123 104
| -0.148 0.134 104
|
| Birthweight | 0.243* 0.013 104
| 1 104
| 0.684** 0.000 104
| 0.485** 0.000 104
| 0.097 0.325 104
| 0.529** 0.000 104
| 0.397** 0.000 104
| 0.046 0.640 104
|
| Length | 0.104 0.294 104
| 0.684** 0.000 104
| 1 104
| 0.489** 0.000 104
| 0.124 0.210 104
| 0.485** 0.000 104
| -0.358** 0.000 104
| 0.080 0.420 104
|
| Head circumference | 0.146 0.139 104
| 0.485** 0.000 104
| 0.489** 0.000 104
| 1 104
| -0.010 0.020 104
| 0.228** 0.282 104
| -0.252** 0.010 104
| -0.015 0.879 104
|
| Total cholesterol | -0.137 0.166 104
| 0.097 0.325 104
| 0.124 0.210 104
| -0.010 0.921 104
| 1 104
| 0.207* 0.035 104
| 0.317** 0.001 104
| 0.968** 0.000 104
|
| Triglycerides | -0.106 0.282 104
| 0.529** 0.000 104
| 0.485** 0.000 104
| 0.228* 0.020 104
| 0.207* 0.035 104
| 1 104
| -0.463** 0.000 104
| 0.052 0.599 104
|
| HDL | 0.152 0.123 104
| -0.397** 0.000 104
| 0.358** 0.000 104
| -0.252** 0.010 104
| 0.317** 0.001 104
| -0.463** 0.000 104
| 1 104
| 0.243* 0.013 104
|
| LDL | -0.148 0.134 104
| 0.046 0.640 104
| 0.080 0.420 104
| -0.015 0.879 104
| 0.968** 0.000 104
| 0.052 0.599 104
| 0.243* 0.130 104
| 1 104
|
*Correlation is significant at the 0.05 level (2-tailed)
**Correlation is significant at the 0.01 level (2-tailed)
Pearson’s correlation was used and correlation was considered as significant at 0.05 level.
Discussion
Between 1980 and 2013, the prevalence rate of childhood overweight and obesity has increased by 47.1%. In developed countries there is notable increase since 1980 i.e., 16.9% boys and 16.2% girls being either overweight and obese compared to 23.8% of boys and 22.6% girls in 2013 [21]. Whereas in developing countries, the prevalence increased from 8.1% in 1980 to 12.9% in 2013 for boys and 8.4% to 13.4% in girls [21]. Childhood obesity and overweight has also become a major health concern in Sri Lanka. According to a study that was conducted in the year 2014, the prevalence of childhood obesity and overweight in the Galle municipal area in Sri Lanka was 10.8% [22]. This rise in childhood obesity and overweight is due to the change in dietary habits and lifestyle due to rapid demographic, nutritional and epidemiological transition. Findings of the present study reveal that the prevalence of LGA babies in a Tertiary Care Center in Southern Sri Lanka is 12.35%. Both prenatal and postnatal factors affect the occurrence of LGA babies. Maternal factors like hypercholesterolaemia, hypertriglyceridaemia, hyperglycaemia and polycythemia and related factors contribute to the birth of LGA babies. So, the birth of LGA babies may be a result of increase in calorie intake and fat intake as well as decline in physical activity of pregnant mothers [9].
Infant development is determined by many factors such as maternal metabolism, gestational weeks at birth, maternal BMI etc., [23]. In this hospital based case-control study, the relationship between maternal lipid markers and anthropometric parameters of newborn infants was determined. It was found that plasma TG concentration but not plasma total cholesterol concentrations, in the third trimester of pregnancy were independently and positively associated with the birth of LGA newborns. TG concentration remained significantly different between LGA and AGA groups. This finding indicated that higher TG level (>150.0 mg/dL) is an independent risk factor for the birth of LGA newborns. Similar results were also reported in a study conducted in Korea which concluded that maternal triglyceride levels were significantly higher in mothers of LGA newborns compared to mothers who gave birth to AGA and SGA newborns and no significant correlations were found between newborn birth weight and maternal total cholesterol level and HDL levels [23]. Results of this study were in line with a research conducted in Amsterdam, which showed that there was a positive correlation between maternal TG levels and birth weight of the newborn. Although, mean levels of TG were significantly higher in mothers who gave birth to LGA newborns, total cholesterol levels were not significantly different [9]. Another similar study in Japan on 146 pregnant mothers showed that mid-pregnancy maternal serum TG was a significant predictor of LGA babies [24]. On the other hand a significant negative correlation was observed between the HDL level and the birth weight (-0.397), birth length (-0.358) and the head circumference (-0.252).
According to literature, mothers with an increased maternal serum triglyceride level have a higher risk for their infant to become LGA. Lipoprotein lipase hydrolyses maternal triglycerides which is important for the foetal development [25,26]. High levels of triglycerides observed in the current study may be due to reduced lipoprotein lipase activity and increased oestrogen concentration observed during the pregnancy period. Schaefer-Graf UM et al., also revealed that maternal triglyceride level influence foetal metabolism and it is highly likely that it will affect foetal and neonatal fat mass [27]. According to this report, triglyceride concentration is strongly correlated with neonatal fat mass/abdominal circumference [27].
However, an association between total cholesterol concentration and the birth of LGA newborns was not observed in this study. The sample of the current study also comprises a relatively healthy population with no association between mothers who gave birth to LGA and AGA newborns in terms of the pre-pregnancy BMI. In the first arm of the study, an association was not found between the birth of LGA babies and the presence of gestational diabetes. According to many published studies gestational diabetes constitutes an important risk factor for macrosomia [28]. The reason why an association between gestational diabetes and the birth of LGA newborns was not found in this study population may be due to proper diabetes control and good antenatal care in the pregnant mothers in Sri Lanka. Sri Lanka has a lower maternal mortality rate and infant mortality rate compared to many developing countries due to improved healthcare in the country. Mothers with gestational diabetes were also excluded from the second arm of the study.
Serum HDL concentration has an important role in reverse cholesterol transport and homeostasis which has an influence on the foetal development. According to McConihay JA et al., although HDL is not thought to cross the placenta, studies done on mice have revealed that a significant difference in the maternal concentration of HDL can affect the sterol metabolism of the yolk sac and the placenta hence the foetal size [29]. In this study, a negative association between HDL concentration and the birth weight of the newborns was observed. Results of the current study were in line with other reports which show negative associations between serum HDL cholesterol and triglyceride levels. Since triglycerides are the essential source of Non Esterified Fatty Acids (NEFAs), it is hypothesised that higher HDL levels in mother might prevent the foetus from the excessive load of NEFA [30].
It was interesting to note that there was no significant difference observed in the LDL concentration between the mothers who gave birth to LGA infants and AGA infants.
Evidence is emerging that adipose tissue function is altered in Asian population even at very low body fat contents. Reports indicate that serum adiponectin concentrations are low in Chinese, Korean and South Asian women compared to other populations [31]. Since adiponectin concentrations are low in metabolic syndrome, low levels of adiponectin observed in the Asian population have drawn more attention over the last few years. South Asians are known to show features of subcutaneous adipose tissues that are similar to those of more obese people. There is evidence to believe that south Asians have higher body fat content for any given BMI [19,32].
This study also has several attractive strengths. This is the first ever study done in Sri Lanka which looked into the association between lipid profile during late pregnancy on the birth weight of the newborn and provided some prospective evidence that dyslipidaemia during pregnancy places Sri Lankan pregnant mothers and their newborns at greater risk of adverse outcomes.
Therefore, findings of this study suggest that high maternal TG and low HDL levels in late pregnancy play a critical role in foetal development and these factors are independently and significantly associated with the birth of LGA infants.
Limitation
However, present study also had some limitations. Unfortunately, the cut off points used in Sri Lanka currently to diagnose obesity are not country specific. Diagnosis of LGA is also done according to CDC guidelines which are not standardised for the Asian population. The sample size used for the study was comparatively small and the study will be continued further to collect more data to obtain a better conclusion. In the future, biochemical parameters such as maternal serum leptin, insulin, C-peptide, and HbA1C levels will be measured to obtain a better understanding about the biochemical changes that would have an effect on the birth of LGA and AGA infants.
Conclusion
There were no association between the birth of LGA infants and the maternal age, monthly income, educational state, occupation of the mother, pregestational BMI and gestational weight gain. However, there were significantly higher levels (p<0.001) of serum triglycerides and significantly lower levels (p<0.001) of serum HDL in mothers who delivered LGA babies compared to mothers who delivered appropriate for gestational age babies. Results of this study show clearly that there is a positive and intermediate correlation between serum triglycerides and the birth of LGA infants but there was a negative and intermediate correlation between HDL concentration and the birth of LGA infants. However, results were not significantly different from the previously published results on other populations hence, this preliminary study suggest that having high body fat in the Sri Lankan population had no significant impact on the birth of LGA infants. Interventional measures should be taken to reduce the adverse effects of high maternal triglycerides on foetal growth and development and the occurrence of LGA babies and their future risk of metabolic syndrome.
Data were collected in the 1st arm of the study (n=149). Data were expressed as n (%). Statistical analysis between large for gestational age (LGA) and appropriate for gestational age (AGA) groups were performed by the chi-square test while maternal age, pre-gestational body mass index (BMI), period of gestation were analysed by Kruskal Wallis test. AGA: Appropriate for gestational age, LGA: Large for gestational age, BMI: Body Mass Index. *p<0.05 was considered significant.
Data was collected in the 1st arm of the study (n=149). Data were expressed as n (%). Statistical analysis between large for gestational age (LGA) and appropriate for gestational age (AGA) groups were performed by the Chi-squared test while maternal age, pre-gestational Body Mass Index (BMI), period of gestation were analysed by Kruskal Wallis test. *p<0.05 was considered significant.
Data was collected in the 2nd arm of the study (n=104). Data were presented as median (interquartile range). Statistical analysis was performed by the Kruskal Wallis test for the differences between the large for gestational age (LGA) and appropriate for gestational age (AGA) groups. *p<0.001.
*Correlation is significant at the 0.05 level (2-tailed)**Correlation is significant at the 0.01 level (2-tailed)Pearson’s correlation was used and correlation was considered as significant at 0.05 level.