Introduction
Innate and adaptive immune responses against bacterial and parasitic infections are still not fully understood, due to many complications during these infections as well as unlimited production of different immune factors in human body. Staphylococcus aureus and T. vaginalis are the most dangerous pathogens cause different infections in women such as vaginal abscess and abortion. Staphylococcus aureus is Gram-Positive bacteria, non-motile, coagulase positive and cause different human illnesses such as bacteremia, burn and soft tissue infections due to the high resistance to different antimicrobial agents and environmental stress and is able to produce several virulence factors. Staphylococcus aureus became a serious public health concern [1,2]. Recently, skin infections caused by S. aureus has increased rapidly [3,4]. Trichomonas vaginalis is a urogenital tract pathogen of humans causing a sexually transmitted diseases in the world, patients infected with skin abscess have increased colonization and infection with S.aureus, and T.vaginalis which has been responsible for decreased antimicrobial peptidase and Th2 cytokine profile such as IL-10, IL-13 and IL-4 [5-7]. Prostaglandin E2 (PGE2) is an important pro-inflammatory biomarker and there is a positive relationship between S.aureus growth and adherence and increase production of PGE2 level in blood of patients infected with skin abscess [8]. Transforming Growth Factor Beta 1 (TGF-β1) is a polypeptide member which has important role in control of cell growth, cell proliferation and cell differentiation in human [9,10]. Lactoferrin is a glycoprotein with a molecular mass 80000 Dalton and has an a multidimentional role in the human body. Also known as lactotrasferrin, it is a part of innate immune system and has antimicrobial activity against different pathogens [11,12]. Trichomonas vaginalis is one of the most important sexually transmitted protozoans in the world [13], with an estimated 170 million cases occurring each year, the epidemiology of the disease is still poorly understood and some practitioners continue to question its importance, it is one of the most common causes of non-viral sexually transmitted diseases in the world [14]. Trichomonas vaginalis infection typically elicits aggressive local cellular immune responses with inflammation of the vaginal epithelium in women and the urethra in men [15]. It can also be transmitted to neonates during passage through an infected birth canal, but the infection is usually asymptomatic [16]. Symptomatic women with trichomoniasis usually complain of vaginal discharge, vulvovaginal soreness or irritation [17]. In Iraq, there are no studies focusing on vaginal abscess caused by S. aureus and T. vaginalis in pregnant women and immune response against these pathogens. Therefore, the aim of this study was the evaluation of four biomarker levels; IL-13, PGE2, TGF-β1 and lactoferrin in serum of pregnant women infected with vaginal abscess and evaluation of the immune response activity of these women.
Materials and Methods
Study Design and Pregnant Women
This is a case control study done in AL-Zahra hospital in AL-Najaf City, Iraq, during the period from January 2017 to November 2017. 120 pregnant women (age range between 20 to 40 years old) infected with acute vaginal abscess (two weeks duration of infection) were included in this study divided into three groups as follow: Group one; 30 pregnant women infected with S.aureus, Group two; 30 pregnant women infected with T. vaginalis, Group three; 30 pregnant women infected with both S.aureus and T. vaginalis. All these groups were compared with 30 healthy pregnant women as control.
Diagnosis of Staphylococcus Aureus
Non-duplicated high vaginal swab (Oxoid, UK) were collected from pregnant women (by specialist physician) infected with acute vaginal abscess and immediately streaked onto blood agar and mannitol salt agar surface (Oxoid, UK) and incubated at 37°C overnight. All growing colonies were identified according to standard laboratory methods [18].
Diagnosis of T. Vaginalis
Non-duplicated samples of vaginal discharge were collected from pregnant women (by specialist physician) infected with acute vaginal abscess. T. vaginalis was identified by wet mount smear as follows: one drop was taken from vaginal discharge and examined by microscope using 40x power object to identify T. vaginalis according to general characters [19].
Serum Collection
Five mL of blood was collected from healthy and infected pregnant women. Blood samples were drawn in sterile vacutainer tubes and left at room temperature for 30 min. Centrifugation was done at 3000 rpm for 5 minute (Memmert, Germany). Serum was collected and kept in sterile tubes at deep freeze at -4°C until use.
Serum Biomarkers Detection
Four human biomarkers were used in this study: IL-13, PGE2, TGF-β1 and lactoferrin. All these biomarker kits were procured from Elabscience Company, Bulgaria and the level of biomarkers in serum were determined by using ELISA device (Human reader, Germany) according to Manufacturer Company as follows: 100μL of standard or sample to each well (96 wells of micro ELISA plate) were added, all liquids were removed, 100μL Biotinylated (detection antibody specific for human IL-13, PGE2, TGF-β1 and lactoferrin) were added and incubated for 1 hour at 37°C then washed 3 times. A 100μL of Avidin-Horseradish Peroxidase (HRP) Conjugate was added and Incubated for 30 minute at 37°C then washed 5 times. A 90μL of substrate reagent was added and incubated for 15 minute at 37°C. A 50 μL of stop solution was added. Finally, the optical density was read at 450 nm immediately and the results were calculated [20].
Statistical Analysis
T-test was used in this study for comparison between samples by using Graph-pad prism version 10 computer software. A p-value less than 0.05 considered statistically significant [21].
Results
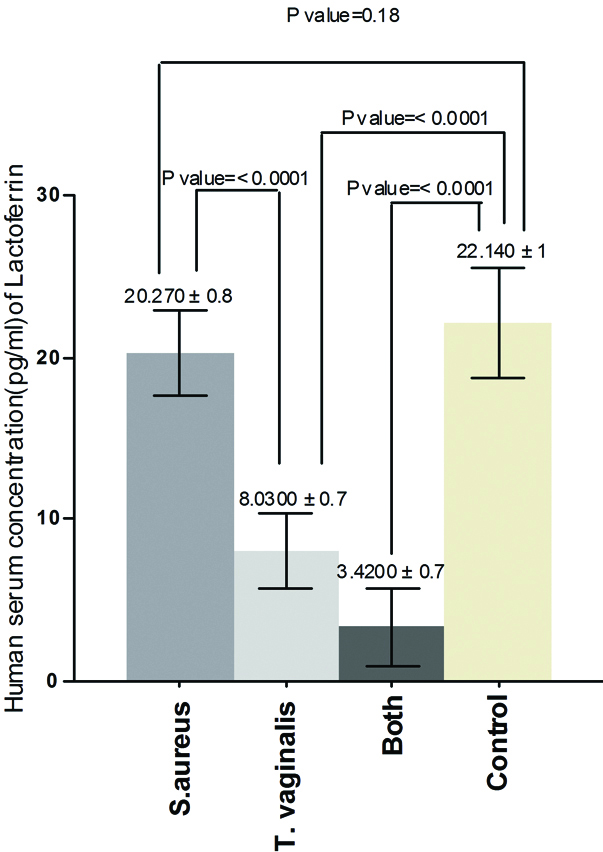
The results of the current study demonstrated that there was significant increase in serum concentration of IL-13 between Group one and control (p-value=0.0001), between Group two and control (p-value=<0.0001), between Group one and Group two (p-value= 0.0059) and between Group three and control (p-value = <0.0001) [Table/Fig-1]. Also, the results proved that there was significant increase in serum concentration of PGE2 between Group one and control (p-value = 0.0092), between Group two and control (p-value = 0.0003) and there was no significant increase between Group one and Group two (p-value = 0.15) but there was significant increase between Group three and control (p-value = 0.0001) [Table/Fig-2]. On the other hand, the results showed that there was significant increase in human serum concentration of TGF-β1 between Group one and control (p-value=0.02), between Group two and control (p-value=0.0011) and there was no significant increase between Group one and Group two (p-value=0.13) but there was significant increase between Group three and control (p-value=0.0007) [Table/Fig-3]. Finally, the results indicated that there was no significant increase in human serum concentration of lactoferrin (p-value=0.18) between Group one and control but there was significant decrease between Group two and control and between Group one and Group two (p-value=<0.0001) also, there was significant decrease between Group three and control (p-value=0.0001) [Table/Fig-4].
Human serum concentration (pg/mL) of IL-13 in pregnant women infected with acute vaginal abscess caused by S. aureus and T. vaginalis.
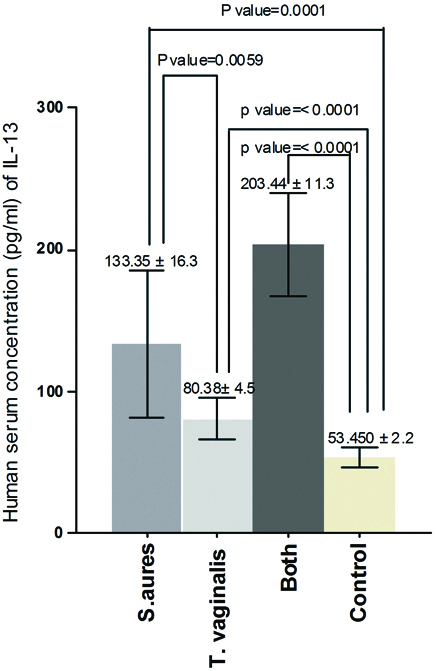
Human serum concentration (pg/mL) of PGE2 in pregnant women infected with acute vaginal abscess caused by S. aureus and T. vaginalis.
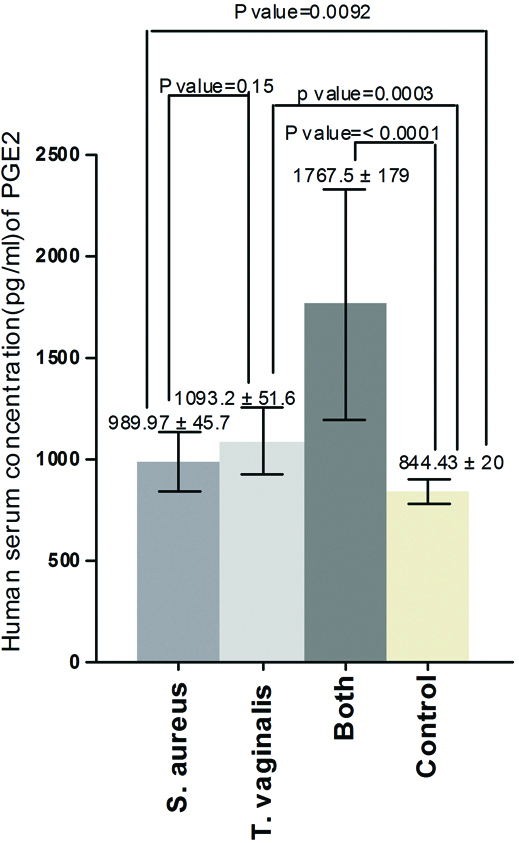
Human serum concentration (pg/mL) of TGF-β1 in pregnant women infected with acute vaginal abscess caused by S. aureus and T. vaginalis.
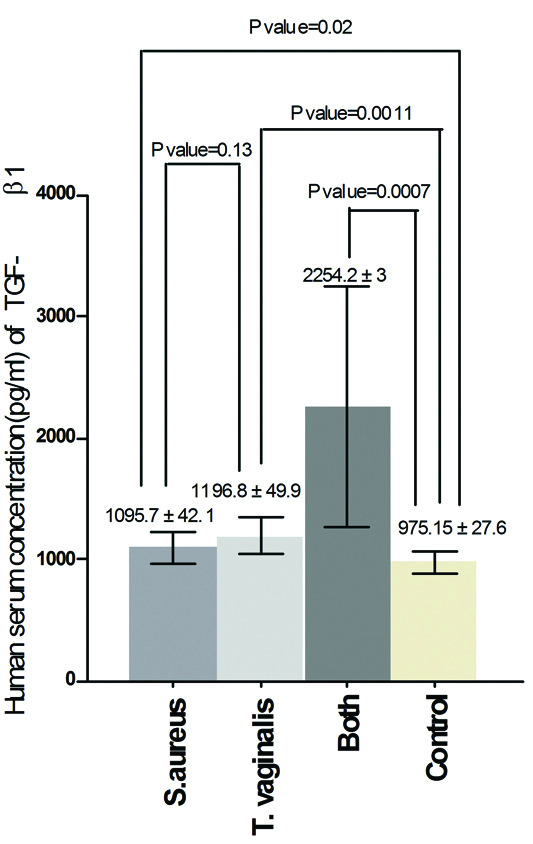
Human serum concentration (pg/mL) of lactoferrin in pregnant women infected with acute vaginal abscess caused by S. aureus and T. vaginalis.
Discussion
This is the first study in Iraq focused on the levels of four biomarkers in serum of pregnant women infected with acute vaginal abscess caused by two different pathogens. In this study, IL-13, PGE2 and TGF-β1 levels were significantly increased (p-value<0.05) in serum of pregnant women infected with acute vaginal abscess caused by S.aureus and T.vaginalis as compared to the control. Staphylococcus aureus and T.vaginalis are two of the most important pathogens which produce several molecules that also contribute to the formation of abscesses in pregnant women [22-24]. IL 13 is a cytokine produced by different cell types such as T-cell and mast cell and responsible for a wide range of biology and functions in the body. Almost, all Th2 cytokines are known to mediate host defense against parasites and bacterial infections but, these cytokines can also trigger infection if their activities are deregulated [25,26]. During bacterial and parasitic infections, IL-13 is able to activate different types of immune cells such as eosinophils and basophils to eliminate bacterial cells during and post infection and activated B-cell to produce IgE (anti-parasitic antibody) [27]. Also, IL-13 has been shown to induce a potent fibrogenic program during the atopic dermatitis, therefore, it has been suggested this fibrogenic program is closely related with production of IL-13 [28]. On the other hand, IL-13 has been identified as a major pathogenic cytokine in acute and chronic disease associated with persistent type2 cytokines production such as IL-4, IL-9 and IL-5 [29,30]. These cytokines are responsible for wound healing after acute tissue injury [31,32]. Therefore, in this study, the level of IL-13 was in high significant increase in serum of pregnant women infected with acute vaginal abscess caused by S.aureus and T.vaginalis. Overuse of antimicrobials to treat of bacterial infections lead to the growing incidence of antibiotic resistant S.aureus and other pathogens. Therefore, that leads to over production of most immune biomarkers such as PGE2, is an oxygenated metabolite of arachidonic acid, PGE2 can be produced^ by all cell types of the body that have epithelia, fibroblast, and infiltrating^ inflammatory cell that represent the major source of PGE2 in the immune response as a response to infection and has different functional versatility [33,34]. In addition, PGE2 act as a pro-inflammatory mediator activating mast cell, macrophages and neutrophils in acute infection stage [35,36]. Some previous studies suggested that PGE2 has a strong suppressor action against innate and adaptive immunity at the molecular and cellular levels [37,38]. While, in other separate studies proved that PGE2 is facilitated the growth and adherence of S.aureus and T.vaginalis and was responsible for colonization of these pathogens, this results are in agreement with result of this study when demonstrated that there was high significant increase of PGE2 in serum of pregnant women infected with S.aureus and T.vaginalis as compare with control. TGF-β1 is a protein, first discovered in platelets of human containing and 390 amino acids with molecular mass 25,000 Dalton and has an important role in wound healing, controlling of immune system, proliferation, differentiation and activation of different cell types [39,40]. TGF-β1 Is secreted by leukocytes and most immune cells such as T-cell [41,42]. In the present study, TGF-β1 was in high significant increase in serum of pregnant women infected with S.aureus and T.vaginalis. Transforming growth factor β1 is able to promote S.aureus adhesion to and invasion into mammary fibroblasts by increasing collagen I and Alpha-Smooth Muscle Actin (β-SMA) expression and provide a novel target for controlling of infection [43]. Previous studies showed that T. vaginalis is able to activate caspase protein-3 and reduce expression of Mcl-1 gene lead to neutrophilic apoptosis during vaginal infection in women [44,45]. As a proven scientific fact, the uptake of apoptotic cells activates the produce of anti-inflammatory biomarkers such as PGE2, TGF-β and Interlukine-10; on the other hand, the uptake of apoptotic cells inhibits the secretion of pro-inflammatory mediators such as Tumour necrotic factor-β from phagocytic cells [46-48]. Lactoferrin is one of the most important components of human immune system serve as antibacterial and anti-parasitic and belongs to iron-binding system [49,50]. Iron is an essential element which has a significant effect on the virulence of T. vaginalis [51]. In this study, the decrease of lactoferrin level in women with T. vaginalis may be due to the pathogenicity of this parasite dependent on the positive relationship between iron concentration and adhesion of parasite on epithelial cell [52,53]. The decrease in lactoferrin levels may be due to an increase in consuming iron by this parasite and this leads to decrease in the storage of iron as ferritin or increased utilization by parasite [54]. The result of the current study is in agreement with the study by Weinberg [55] who suggested that the T. vaginalis uses the ferritin as source of iron which leads to decrease in the ferritin level in serum of women infected with T. vaginalis compared with healthy control. Enzymatic degradation is one of the important mechanisms was used by the parasite to proteases secretion and release iron from lactoferrin as a source of nutrition [56].
Limitation
We think this study has reached its aim, but there were some unavailable limitations such as study the level of other biomarkers such as CD4+ and CD8+ in the same patients.
Conclusion
The personal hygiene plays an important role to prevent wide range of sexually transmitted disease caused by different pathogens. The synergistically pathogenic effect of S.aureus and T.vaginalis in vagina of pregnant women infected with acute vaginal abscess lead to activation of T-cell and overproduction of IL-3, PGE2 and TGF-β1 as a good signaling markers against infection. In contrast, this synergistic effect leads to reduction of lactoferrin levels in the same women. These women may be susceptible to acute anemia that may be lead to abortion. Therefore, the individuals can protect themselves from different sexually transmitted diseases by paying attention to personal hygiene and use a condom.
[1]. Subramanian A, Chitalia VK, Bangera K, Vaidya SP, Warke R, Chowdhary A, Evaluation of hiaureus TM coagulase confirmation kit in identification of Staphylococcus aureusJ Clin Diagn Res 2017 11(2):DC08-DC13.10.7860/JCDR/2017/24021.926528384859 [Google Scholar] [CrossRef] [PubMed]
[2]. Das R, Sebastian S, Kapil A, Dhawan B, Decline of nosocomial methicillin-resistant staphylococcus aureus skin and soft tissue infections in an Indian tertiary hospital: hope for the futureJ Clin Diagn Res 2017 11(8):DL01-DL02.10.7860/JCDR/2017/27677.1034428969128 [Google Scholar] [CrossRef] [PubMed]
[3]. Aljanaby AAJ, Antibacterial activity of an aqueous extracts of Alkanna tinctoria roots against drug resistant aerobic pathogenic bacteria isolated from patients with burns infectionsROMJ 2018 7(1):01-06.10.15275/rusomj.2018.0104 [Google Scholar] [CrossRef]
[4]. Jiang H, Zou J, Cheng H, Fang J, Huang G, Purification, characterization, and mode of action of pentocin jl-1, a novel bacteriocin isolated from lactobacillus pentosus, against drug-resistant staphylococcus aureusBiomed Res Int 2017 2017(1):765719010.1155/2017/765719029333451 [Google Scholar] [CrossRef] [PubMed]
[5]. Bieber T, Atopic dermatitisN Engl J Med 2008 358(14):1483-94.10.1056/NEJMra07408118385500 [Google Scholar] [CrossRef] [PubMed]
[6]. Cho SH, Strickland I, Tomkinson A, Fehringer AP, Gelfand EW, Leung DY, Preferential binding of Staphylococcus aureus to skin sites of Th2-mediated inflammation in a murine modelJ Invest Dermatol 2001 116(5):658-63.10.1046/j.0022-202x.2001.01331.x11348452 [Google Scholar] [CrossRef] [PubMed]
[7]. Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Endogenous antimicrobial peptides and skin infections in atopic dermatitisN Engl J Med 2002 347(15):1151-60.10.1056/NEJMoa02148112374875 [Google Scholar] [CrossRef] [PubMed]
[8]. Wang Y, Ren B, Zhou X, Liu S, Zhou Y, Li B, Growth and adherence of Staphylococcus aureus were enhanced through the PGE2 produced by the activated COX-2/PGE2 pathway of infected oral epithelial cellsPLoS One 2017 12(5):e017716610.1371/journal.pone.017716628472126 [Google Scholar] [CrossRef] [PubMed]
[9]. Ghadami M, Makita Y, Yoshida K, Nishimura G, Fukushima Y, Wakui K, Genetic mapping of the Camurati-Engelmann disease locus to chromosome 19q13.1-q13.3Am J Hum Genet 2000 66(1):143-47.10.1086/30272810631145 [Google Scholar] [CrossRef] [PubMed]
[10]. Vaughn SP, Broussard S, Hall CR, Scott A, Blanton SH, Milunsky JM, Confirmation of the mapping of the Camurati-Englemann locus to 19q13. 2 and refinement to a 3.2-cM regionGenomics 2000 66(1):119-21.10.1006/geno.2000.619210843814 [Google Scholar] [CrossRef] [PubMed]
[11]. Sánchez L, Calvo M, Brock JH, Biological role of lactoferrin"Arch Dis Child 1992 67(5):657-61.10.1136/adc.67.5.6571599309 [Google Scholar] [CrossRef] [PubMed]
[12]. Levin RE, Kalidas S, Gopinadhan P, Pometto A, Food biotechnology 2006 Boca Raton, FL: CRC/Taylor & Francis:1028ISBN 0-8247-5329-1 [Google Scholar]
[13]. Hui BB, Reulein CP, Guy RJ, Donovan B, Hocking JS, Law MG, Impact of replacing cytology with human papillomavirus testing for cervical cancer screening on the prevalence of Trichomonas vaginalis: a modelling studySex Transm Infect 2018 pii:sextrans-2017-05329410.1136/sextrans-2017-05329429326178 [Google Scholar] [CrossRef] [PubMed]
[14]. Menezes CB, Frasson AP, Tasca T, Trichomoniasis-are we giving the deserved attention to the most common non-viral sexually transmitted disease worldwide?Microb Cell 2016 3(9):404-19.10.15698/mic2016.09.52628357378 [Google Scholar] [CrossRef] [PubMed]
[15]. Shafir SC, Sorvillo FJ, Smith L, Current issues and considerations regarding trichomoniasis and human immunodeficiency virus in African-AmericansClin Microbiol Rev 2009 22(1):37-45.10.1128/CMR.00002-0819136432 [Google Scholar] [CrossRef] [PubMed]
[16]. Crucitti T, Jespers V, Mulenga C, Khondowe S, Vandepitte J, Buvé A, Non-sexual transmission of Trichomonas vaginalis in adolescent girls attending school in Ndola, ZambiaPLoS One 2011 6(1):e1631010.1371/journal.pone.001631021305023 [Google Scholar] [CrossRef] [PubMed]
[17]. Grover S, Avasthi A, Gupta S, Hazari N, Malhotra N, Do female patients with nonpathological vaginal discharge need the same evaluation as for Dhat syndrome in males?Indian J Psychiatry 2016 58(1):61-69.10.4103/0019-5545.17437626985107 [Google Scholar] [CrossRef] [PubMed]
[18]. Aljanaby AAJJ, Antibacterial activity of an aqueous extract of Petroselinum crispum leaves against pathogenic bacteria isolated from patients with burns infections in Al-najaf Governorate, IraqRes Chem Intermed 2012 38(9)10.1007/s11164-012-0874-5 [Google Scholar] [CrossRef]
[19]. World Health OrganizationGuidelines for the management of sexually transmitted infectionsWorld Health Organization 2003 [Google Scholar]
[20]. Broughton SE, Dhagat U, Hercus TR, Nero TL, Grimbaldeston MA, Bonder CS, The GM–CSF/IL-3/IL-5 cytokine receptor family: from ligand recognition to initiation of signalingImmunological Reviews 2012 250(1):277-302.10.1111/j.1600-065X.2012.01164.x23046136 [Google Scholar] [CrossRef] [PubMed]
[21]. Aljanaby AAJ, Alhasnawi HMRJ, Phenotypic and molecular characterization of multidrug resistant klebsiella pneumoniae isolated from different clinical sources in Al-Najaf Province-IraqPak J Biol Sci 2017 20(5):217-32.10.3923/pjbs.2017.217.23229023034 [Google Scholar] [CrossRef] [PubMed]
[22]. Aljanaby AAJ, Aljanaby IAJ, Profile of antimicrobial resistance of aerobic pathogenic bacteria isolated from different clinical infections in Al-Kufa Central Hospital–Iraq During period from 2015 to 2017Research J Pharma and Tech 2017 70(10):3264-16.10.5958/0974-360X.2017.00579.0 [Google Scholar] [CrossRef]
[23]. Bhattacharya S, Pal K, Jain S, Chatterjee SS, Konar J, Surgical site infection by methicillin resistant staphylococcus aureus- on decline?J Clin Diagn Res 2016 10(9):DC32-DC36.10.7860/JCDR/2016/21664.858727790436 [Google Scholar] [CrossRef] [PubMed]
[24]. Hamilton H, Pontiff KL, Bolton M, Bradbury RS, Mathison BA, Bishop H, Trichomonas vaginalis brain abscess in a neonateClin Infect Dis 2018 66(4):604-07.10.1093/cid/cix90829069338 [Google Scholar] [CrossRef] [PubMed]
[25]. May RD, Fung M, Strategies targeting the IL-4/IL-13 axes in diseaseCytokine 2015 75(1):89-116.10.1016/j.cyto.2015.05.01826255210 [Google Scholar] [CrossRef] [PubMed]
[26]. Valizadeh A, Khosravi A, Zadeh LJ, Parizad EG, Role of IL-25 in ImmunityJ Clin Diagn Res 2015 9(4):OE01-04.10.7860/JCDR/2015/12235.581426023586 [Google Scholar] [CrossRef] [PubMed]
[27]. Olmos-Ortiz LM, Barajas-Mendiola MA, Barrios-Rodiles M, Castellano LE, Arias-Negrete S, Avila EE, Trichomonas vaginalis exosome-like vesicles modify the cytokine profile and reduce inflammation in parasite-infected miceParasite Immunol 2017 39(6)10.1111/pim.1242628345149 [Google Scholar] [CrossRef] [PubMed]
[28]. Gieseck RL, Ramalingam TR, Hart KM, Vannella KM, Cantu DA, Lu WY, Interleukin-13 activates distinct cellular pathways leading to ductular reaction, steatosis, and fibrosisImmunity 2016 45(1):145-58.10.1016/j.immuni.2016.06.00927421703 [Google Scholar] [CrossRef] [PubMed]
[29]. Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA, An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory responseJ Clin Invest 1999 104(6):777-85.10.1172/JCI732510491413 [Google Scholar] [CrossRef] [PubMed]
[30]. Wynn TA, Type 2 cytokines: mechanisms and therapeutic strategiesNat Rev Immunol 2015 15(5):271-82.10.1038/nri383125882242 [Google Scholar] [CrossRef] [PubMed]
[31]. Kaviratne M, Hesse M, Leusink M, Cheever AW, Davies SJ, McKerrow JH, IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independentJ Immunol 2004 173(6):4020-29.10.4049/jimmunol.173.6.402015356151 [Google Scholar] [CrossRef] [PubMed]
[32]. Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infectionNat Med 2012 18(2):260-66.10.1038/nm.262822245779 [Google Scholar] [CrossRef] [PubMed]
[33]. Park JY, Pillinger MH, Abramson SB, Prostaglandin E2 synthesis and secretion: the role of PGE 2synthasesClin Immunol 2006 119(3):229-40.10.1016/j.clim.2006.01.01616540375 [Google Scholar] [CrossRef] [PubMed]
[34]. Hata AN, Breyer RM, Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulationPharmacol Ther 20014 103:147-66.10.1016/j.pharmthera.2004.06.00315369681 [Google Scholar] [CrossRef] [PubMed]
[35]. Nakayama T, Mutsuga N, Yao L, Tosato G, Prostaglandin E2 promotes degranulation-independent release of MCP-1 from mast cellsJ Leukoc Biol 2006 79(1):95-104.10.1189/jlb.040522616275896 [Google Scholar] [CrossRef] [PubMed]
[36]. Kalinski P, Regulation of immune responses by prostaglandin E2J Immunol 2012 188(1):21-28.10.4049/jimmunol.110102922187483 [Google Scholar] [CrossRef] [PubMed]
[37]. Harris SG, Padilla J, Koumas L, Ray D, Phipps RP, Prostaglandins as modulators of immunityTrends Immunol 2002 23(3):144-50.10.1016/S1471-4906(01)02154-8 [Google Scholar] [CrossRef]
[38]. Obermajer N, Wong JL, Edwards RP, Odunsi K, Moysich K, Kalinski P, PGE2-driven induction and maintenance of cancer-associated myeloid-derived suppressor cellsImmunol Invest 2012 41(6-7):635-57.10.3109/08820139.2012.69541723017139 [Google Scholar] [CrossRef] [PubMed]
[39]. Kucknoor A, Mundodi V, Alderete JF, Trichomonas vaginalis adherence mediates differential gene expression in human vaginal epithelial cellsCell Microbiol 2005 7(6):887-97.10.1111/j.1462-5822.2005.00522.x15888089 [Google Scholar] [CrossRef] [PubMed]
[40]. Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB, Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterizationJ Biol Chem 1983 258(11):7155-60. [Google Scholar]
[41]. Derynck R, Jarrett JA, Chen EY, Eaton DH, Bell JR, Assoian RK, Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cellsNature 1985 316(6030):701-05.10.1038/316701a03861940 [Google Scholar] [PubMed] [PubMed]
[42]. Letterio JJ, Roberts AB, Regulation of immune responses by TGF-betaAnnu Rev Immunol 1998 16:137-61.10.1146/annurev.immunol.16.1.1379597127 [Google Scholar] [CrossRef] [PubMed]
[43]. Travis MA, Sheppard D, TGF-β activation and function in immunityAnnu Rev Immunol 2014 32:51-82.10.1146/annurev-immunol-032713-12025724313777 [Google Scholar] [CrossRef] [PubMed]
[44]. Zhao S, Gao Y, Xia X, Che Y, Wang Y, Liu H, TGF-β1 promotes Staphylococcus aureus adhesion to and invasion into bovine mammary fibroblasts via the ERK pathwayMicrob Pathog 2017 106:25-29.10.1016/j.micpath.2017.01.04428131949 [Google Scholar] [CrossRef] [PubMed]
[45]. Kang JH, Song HO, Ryu JS, Shin MH, Kim JM, Cho YS, Trichomonas vaginalis promotes apoptosis of human neutrophils by activating caspase-3 and reducing Mcl-1 expressionParasite Immunol 2006 28(9):439-46.10.1111/j.1365-3024.2006.00884.x16916367 [Google Scholar] [CrossRef] [PubMed]
[46]. Song HO, Shin MH, Ahn MH, Min DY, Kim YS, Ryu JS, Trichomonas vaginalis: Reactive oxygen species mediates caspase3 dependent apoptosis of human neutrophilsParasite Immunol 2008 118:59-65.10.1016/j.exppara.2007.06.01017709105 [Google Scholar] [CrossRef] [PubMed]
[47]. Volle RE, Herrmann M, Roth EA, Stach C, Kalden JR, Immunosuppressive effects of apoptotic cellsNature 1997 390:350-51.10.1038/370229389474 [Google Scholar] [CrossRef] [PubMed]
[48]. Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM, Macrophage that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAFJ Clin Invest 1998 101:890-98.10.1172/JCI11129466984 [Google Scholar] [CrossRef] [PubMed]
[49]. Huynh ML, Fadok VA, Henson PM, Phosphatidylser inedependent ingestion ofapoptotic cells promotes TGF-β1 secretion and the resolution of inflammationJ Clin Invest 2002 109:41-50.10.1172/JCI021163811781349 [Google Scholar] [CrossRef] [PubMed]
[50]. Lopez AF, Williamson DJ, Gamble J, Begley GG, Harlan J, Klebanoff S, Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survivalJCI 1986 78:122010.1172/JCI1127053021817 [Google Scholar] [CrossRef] [PubMed]
[51]. Al-Hadraawy SK, Al-ghurabi ME, Almusawi MM, Alzeyadi M, Ghrelin and melatoninas biomarkers in patients with giardiasisJ Biotechnology & Biotechnological Equipment 2016 30(3):553-57.10.1080/13102818.2016.1149038 [Google Scholar] [CrossRef]
[52]. Ryu JS, Choi HK, Min DY, Ha SE, Ha MH, Ahn MH, Effect of iron on the virulence of Trichomonas vaginalisJ Parasitol 2001 87(2):457-60.10.1645/0022-3395(2001)087[0457:EOIOTV]2.0.CO;2 [Google Scholar] [CrossRef]
[53]. García JR, Krause A, Schulz S, Rodríguez-Jiménez FJ, Klüver E, Adermann K, Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activityFASEB J 2001 15(10):1819-21.10.1096/fj.00-0865fje11481241 [Google Scholar] [CrossRef] [PubMed]
[54]. Saleem Khteer Al-Hadraawy SK, Study role of melatonin and leptin in patient infectedwith Entamoeba histolyticaResearch J Pharm and Tech 2017 10(10):1827-30.10.5958/0974-360X.2017.00620.5 [Google Scholar] [CrossRef]
[55]. Kim YS, Song HO, Choi IH, Park SJ, Ryu JS, Hydrogenosomal activity of Trichomonas vaginalis cultivated under different iron conditionsKorean JParasitol 2006 44:373-78.10.3347/kjp.2006.44.4.37317170580 [Google Scholar] [CrossRef] [PubMed]
[56]. Weinberg ED, The role of iron in protozoan and fungal infectious diseaseJ. Eukaryote. Microbiol 1999 46(1):231-38.10.1111/j.1550-7408.1999.tb05119.x10377984 [Google Scholar] [CrossRef] [PubMed]