Rising incidence of drug resistance resulting in total treatment failure in health care setting is a matter of great concern now to the clinicians. Tuberculosis is a highly infectious disease and the concern has increased rapidly due to the emergence of MDR (Multi Drug Resistant) and XDR (Extensively Drug Resistant) strains of M. tuberculosis [1]. Previous studies had shown that screening of natural molecules have always led to the discovery of several novel antimicrobial agents and those compounds were categorised as secondary metabolites [2]. Natural compounds both in pure compound form and also as a standardised extract formulations acts as a reservoir of new pharmaceutical agents due to its diverse chemical complexity [3]. In case of tuberculosis, the problem has become worse not only due to the emergence of drug resistance strains but also due to the persistence of Mycobacterium. The major drawbacks of the treatment process of tuberculosis are basically long term therapy and several health discomforts which often generate poor patient compliance [3]. Interestingly, besides various usual contributing factors adds to the risk of suffering from tuberculosis, lung which acts as most important silicon accumulation in human body and its silicon content might also play a role in the increased pathogenicity of M. tuberculosis in cases of lung tuberculosis [4].
Many medicinal plants have already been explored for their antituberculous activity as evident from our literature survey but there is no report regarding the antituberculosis activity of mushrooms. Antibacterial activity of crude extracts of mushrooms against several common pathogens and also their activities synergistically with classical antibiotics have revealed its immense therapeutic potential against multidrug resistant bacteria [5-9].
Synergy is considered to be one of the governing principles of nature and is considered to be one of the foremost reasons for the growing complexity of the evolutionary process. The word itself denotes that “the whole is greater than the sum of its parts” [10]. Therefore this study was also aimed to analyse the synergistic antituberculosis activity of an edible mushroom extract with some common antituberculous drugs. Further study on compound characterisation of the ingredients present in the crude extracts which might be responsible for antimycobacterial activity was also taken into account. Haemotoxicity analysis of the crude extract was evaluated as a measure of cytotoxicity. Synergy study with the standard drugs were carried out to evaluate its potency in lowering the higher MIC values of these drugs and in turn lowering the resistance developed against them.
Materials and Methods
Type, Place and Duration of Study
This was an in vitro experimental study. The study was done at Peerless Hospital and BK Roy Research Centre, Kolkata and in Jadavpur University, Kolkata. The duration of the study was 1 year from October 2016 to September 2017.
Mycobacterial Strains Collection
One control strain H37Ra (Mycobacterium tuberculosis H37Ra ATCC 25177™) provided by Bengal Tuberculosis Association, Kolkata and two wild strains of M. tuberculosis and one MOTT (Mycobacterium Other Than Tuberculosis) were isolated from our hospital laboratory from sputum and were used in this study. Wild strains were identified by automated system BD BACTEC™ MGIT™ 320. For further confirmation, the colonial appearances on Lowenstein-Jensen medium and acid fastness by Ziehl-Neelsen staining were also studied time to time during the course of the experiment.
Collection of Edible Mushroom Samples
Fresh edible sample of oyster mushroom Pleurotus ostreatus was collected from reputed mushroom cultivation farm of Ramakrishna Mission, Narendrapur, Kolkata, India [Table/Fig-1]. The mushroom was selected for the study because of its edible nature and also depending upon its growth conditions which matches with the tropical climatic condition of the country. Moreover, the oyster mushroom chosen in this study was reported to have a wide range of medicinal properties such as anticancer, antihyperlipidemic, antihypertensive, antidiabetic, hepatoprotective, antioxidant, antimicrobial and antiallergic [11].
Oyster mushroom Pleurotus ostreatus.

Antibiotics used for the Assay
Standard drugs used for the synergy assay were: Drugs Isoniazid (INH), Ethambutol (EMB), Rifampicin (RIF) which were purchased from Himedia Laboratories Pvt., Ltd., India.
Preparation of Extracts
The fresh mushroom sample was shade dried for 72 hours and powdered using electrical grinder. Solvent extraction process was employed for the extraction of bioactive components. The powdered mushroom sample was extracted using absolute ethanol for 72 hours at dark conditions and at temperature of 30°C. The extract was lyophilized and the crude content was freeze dried for future analysis [12]. Different concentration of the crude extract content was prepared by dissolving the crude content in 10% DMSO (Di Methyl Sulfoxide).
Preparation of Cell Suspension of M. tuberculosis
Preparation of initial inoculum was done by subculturing M. tuberculosis from original growth on L-J medium and it was allowed to reach the log growth phase (approximately upto 12 days as the growth rate of M. tuberculosis is very slow even in subcultures). Then the bacterium (2-3 small isolated colonies) was suspended in 3 ml sterile normal saline (0.9%) solution contained in screw capped Bijou vials having 3-4 glass beads (3mm diameter). The suspension was then vortexed for about 15 minutes using cyclomixer (Remi Equipments CM101) and left to stand for 30 minutes at room temperature. The homogenous supernatant was then adjusted to 1McFarland opacity. Finally 0.1 McFarland opacity suspensions were prepared by further dilution (1:10). 50 μL of this suspension was added to 500 μL of Kirchner media [13].
Minimum Inhibitory Concentration Assay
Minimum Inhibitory concentration (MIC) of the extract was assayed against the collected mycobacterial strains by using Kirchner liquid medium supplemented with antibiotic cocktail Polymixin B, Amphotericin B, Carbenicillin, Trimethoprim (PACT) and finally cultured on Lowenstein-Jensen (LJ) medium. The desired concentration of the extract was achieved by adding the required dry content of the extract to the Kirchner medium for the MIC assay. The range of concentration of crude extract used for the MIC assay were 0.1, 0.5, 1, 2, 4, 8, 10, 12, 15, 20, 25 mg/mL calculated from the dry weight of the crude extract. After inoculation of the set with the standard inoculum, it was kept for 14-21 days incubation at 37°C incubator. After incubation the broth containing culture was inoculated on LJ slant and was again kept in incubation keeping all the other conditions same in ordinary incubator. The control set consisted of only the Kirchner media inoculated with the same amount of bacterial inoculum. One solvent control of DMSO with same standard inoculum was also kept for the assay to note any direct effect of solvent on bacterial growth. Presence or absence of growth with respect to the control set was compared and in this way MIC value was determined by using proportionate method [14].
Haemotoxicity Analysis of the Crude Extracts
Preparation of Erythrocytes: A 10 mL fresh blood sample was collected from a healthy human volunteer (author DC) directly into EDTA coated vacutainer tubes to prevent coagulation. Then the sample was centrifuged at 500 x g for 5 minutes and the levels of haematocrit and plasma was marked. Plasma was gently aspirated with a micropipette and discarded. The haematocrit was filled upto the initial level of plasma with 150 mM sodium chloride (NaCl) solution. The tube was tightly capped and inverted gently few times to mix the contents. Again the sample was centrifuged at 500 x g for 5 minutes. The above step was repeated three to four times to wash the red blood cells. Then the sample was diluted (1:50) with NaCl buffer solution and the haziness was visually inspected [15].
Lysis assay using 96 Well Plate Set up and Quantification
A10 μL of crude extracts at concentration of 10 mg/mL, 15 mg/mL and 20 mg/mL was used for the assay. A 190 μL of diluted erythrocyte suspension was taken and mixed with 10 μL of the extract samples. For positive control 10% Triton X-100 was used and for negative control 150 mM NaCl buffer was used. The samples were incubated at 37°C for one hour. Then the content was centrifuged for 5 minutes at 500 x g to pellet the intact erythrocytes. A 100 μL of clear supernatant fluid was then transferred into flat bottomed 96 well plates. The absorbance of the supernatant was measured at 450 nm using plate reader [15].
Ultra Performance Liquid Chromatography and Mass Spectrometry Conditions for Analysis of Phenol and Flavonoid Compounds
Reversed-phase ultra performance liquid chromatography and electrospray ionization mass spectrometry was used to analyse the phenol and flavonoid contents of the crude extracts. For this purpose Waters Acquity UPLC system and Waters Acquity SQD mass spectrometer (Single quadrupole mass spectrometer) system was used. Chromatographic separations were carried out following these conditions: temperature 50°C; column Resteck (2.1 x 30 mm, 1.8 micron particle size); mobile phase 98% of 0.05% HCOOH (Solvent A) in water and 2% CH3CN (Solvent B), held for 0.75 minute, then to 90% HCOOH (0.05%) in water and 10% CH3CN for 1.0 minute, further to 2% HCOOH(0.05%) in water and 98% CH3CN for 2.0 minute, held this mobile phase composition up to 2.25 min and finally back to initial condition in 3.0 minute. The flow rate was 1.5 mL/minute. Details are given in [Table/Fig-2].
Mobile phase conditions in chromatographic separations.
| Time (minutes) | Module | %A | % B |
|---|
| 0.00 | Pumps | 98 | 2 |
| 0.75 | Pumps | 98 | 2 |
| 1.00 | Pumps | 90 | 10 |
| 2.00 | Pumps | 2 | 98 |
| 2.25 | Pumps | 2 | 98 |
| 2.90 | Pumps | 98 | 2 |
| 3.00 | Pumps | 98 | 2 |
(A=HCOOH; B=CH3CN)
The MS system was equipped with electrospray ionization source operated in the positive ion mode. The following conditions were maintained: Capillary (kV) 3.00; Cone (V) 40.00; Extractor (V) 3.00; Source Temperature (°C) 150; Desolvation Temperature (°C) 400; Cone Gas Flow (L/Hr) 50; Desolvation Gas Flow (L/Hr) 750.
The analytes were identified by comparing the m/z value of the standard compounds mentioned in previous literatures [12,16-21].
Synergy Study with Standard Approved Drugs for the Treatment of Tuberculosis
MIC values of crude extract and the drugs were evaluated individually by dilution method as mentioned above. The MIC values of the drugs were kept from lower to higher dilutions by serial dilution method. The mushroom extract was added finally to all the sets at a final concentration of 7 mg/mL. Then 50 μL 0.1 Mc Farland opacity suspension of Mycobacteria spp. were added to all the different sets. The sets were kept at incubation at 5% CO2 concentration, 37°C for next 7 days. After that 100 μL of culture was inoculated to LJ slants and again kept at same condition of incubation for the next 21 days. The minimum dilution which could restrict the growth of the test organisms in comparison to control set (without antibiotic and mushroom extract) was considered to be MIC for the combined set. The Fractional Inhibitory Concentration Index (FICI) was analysed according to the equation:
MICantibiotic + extract/MICantibiotic [9]. The results were analysed accordingly: (<1) Synergistic; (1-4) Indifference; (>4) Antagonistic. Mycocult kit manufactured by Tulip Diagnostics (P) Ltd was used for the assay. All the assays were carried out in duplicate to assess the reproducibility [16].
Results
Results of this Study are Given in [Table/Fig-3-11]
As shown in [Table/Fig-3,4,5,6,7,8,9,10 and 11] the MIC value of the crude mushroom extract ranges between 1 mg/mL to 10 mg/mL against the reference and clinical isolates of Mycobacteria spp. collected. The absence of haemolytic activity by the crude content of the mushroom extracts can be considered to be an encouraging outcome with respect to clinical application for future study. An array of bioactive components were reported to be present in the crude mushroom extracts which may also correlate with the antimycobacterial activity shown by the mushroom extract alone and in combination with the standard antituberculous drugs such as ethambutol and rifampicin.
The table shows the MIC values (in mg/mL) of the mushroom extract against the strains of Mycobacteria spp.
| Mushroom Samples | M. tuberculosis (ATATCC 25177) H37Ra | M. tuberculosis S1 | M. tuberculosis S2 | MOTT (Mycobacterium other than tuberculosis) |
|---|
| P. ostreatus | 1 | 10 | 10 | 2.5 |
The study was done in triplicates.
The table shows the presence (+) or absence (-) of haemolytic activity of the Triton X and crude ingredients of the mushrooms respectively.
| Concentrations (mg/mL) | P. ostreatus | Triton X-100 (control) |
|---|
| 5 | - | + |
| 10 | - | + |
| 15 | - | + |
| 20 | - | + |
Analysis of LCMS data of the mushroom extracts.
| Sl. No. | Av m/z experimental | Exact Mass | Putative I.D of the compounds | Reference |
|---|
| 1. | 162.0 | 164.16 | p-coumaric acids | [16] |
| 2. | 268.1 | 270.24 | Apigenin | [16] |
| 3. | 330.0 | 330.9 | Gallic-caffeic acid ester | [16] |
| 4. | 222.9 | 224.2 | Sinapic acid | [12] |
| 5. | 463.1 | 464.09 | Isoquercetin/Myricitrin | [19,20] |
| 6. | 612.9 | 610.1534 | Rutin | [19] |
Analysis of synergy combination of mushroom P. ostreatus extract with standard antibiotics based on FICI values.
| Microorganisms | FICI value of Rifampicin | FICI value of Isoniazid | FICI value of Ethambutol |
|---|
| M. tuberculosis H37Ra | 0.078 (S) | 10.08 (A) | 0.833 (S) |
| M. tuberculosis S1 | 0.078 (S) | 2.5 (I) | 0.833 (S) |
| M. tuberculosis S2 | 0.039 (S) | 10 (A) | 0.625 (S) |
| MOTT | 0.004 (S) | 0.004 (S) | 0.128 (S) |
Where A denotes Antagonistic interaction; I denotes Indifference; S denotes Synergy.
(X axis: Time in minutes; Y axis: Absorbance Unit, DAD Wavelength range: 210-400 nm). UPLC profile of bioactive components present in the extract of mushroom P. ostreatus showing the retention time (RT), Area, % Area and height.
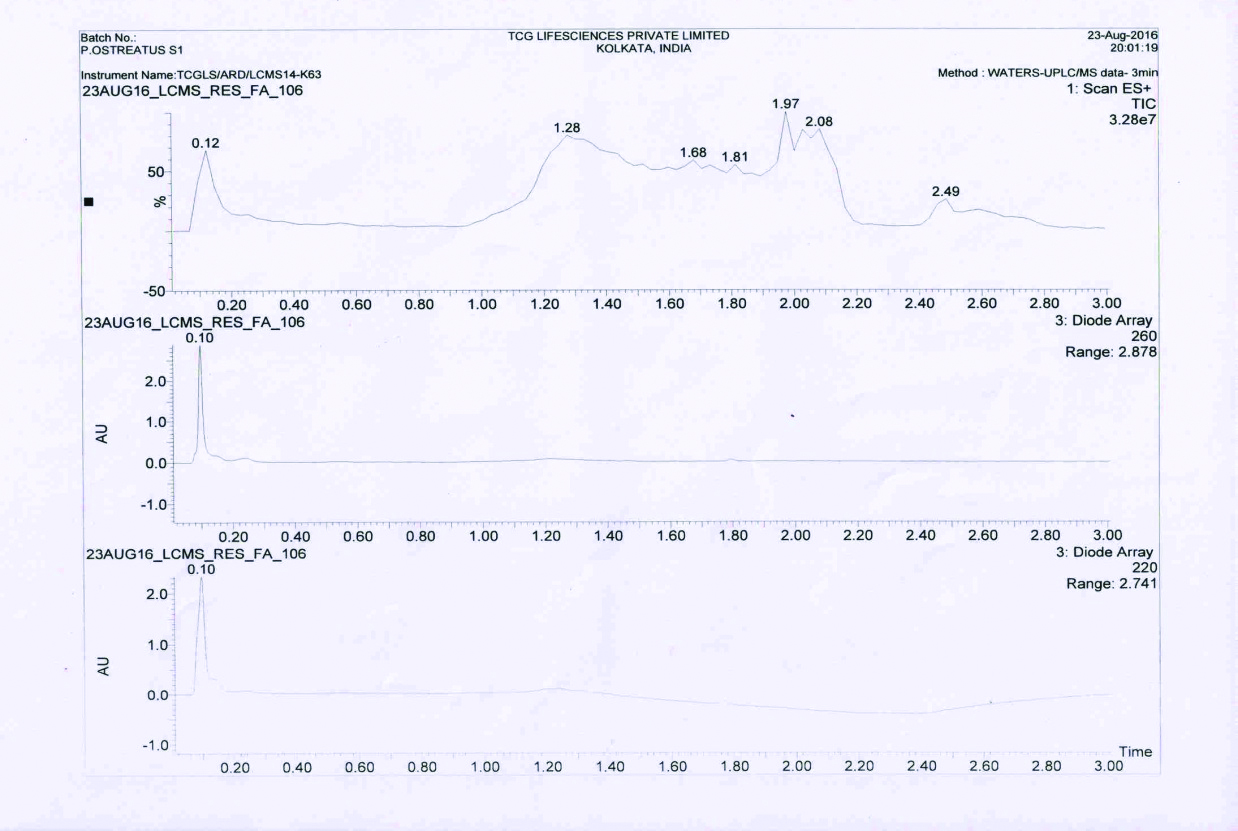
(X axis: mass/charge (m/z); Y axis: Relative abundance (%)). The figure represents the MSn fragmentation of representative compounds of the extract of mushroom P. ostreatus.
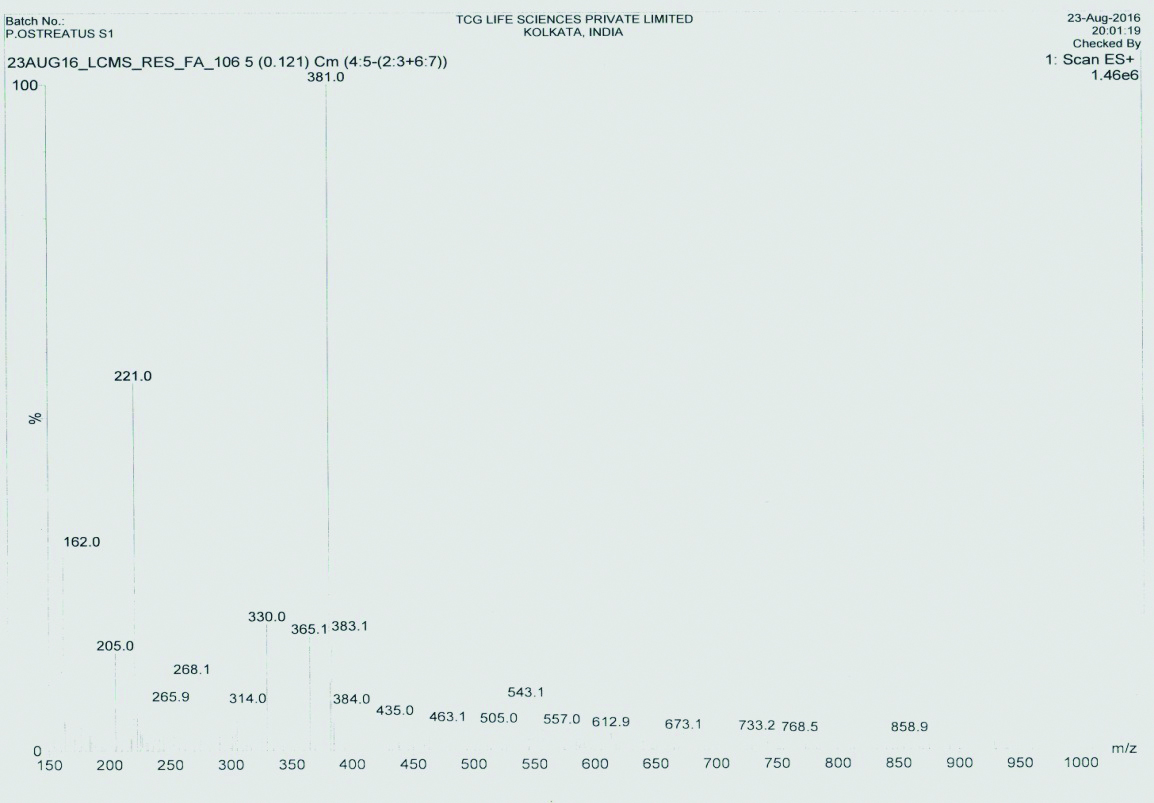
The bar diagram represents the mean of MIC values (in μg/mL) of the ethambutol alone and in combination with the extract of mushroom P. ostreatus.
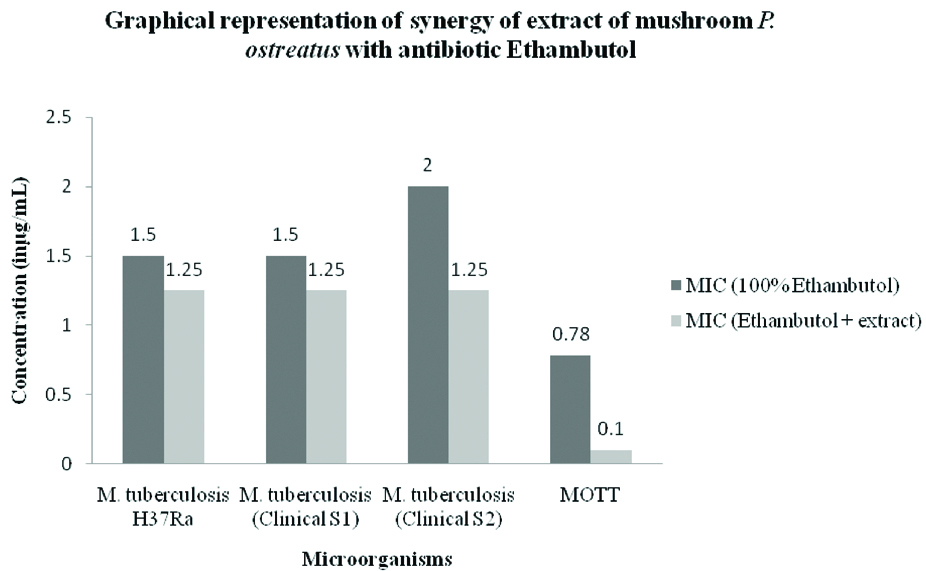
The bar diagram represents the mean of MIC values (in μg/mL) of the isoniazid alone and in combination with the extract of mushroom P. ostreatus.
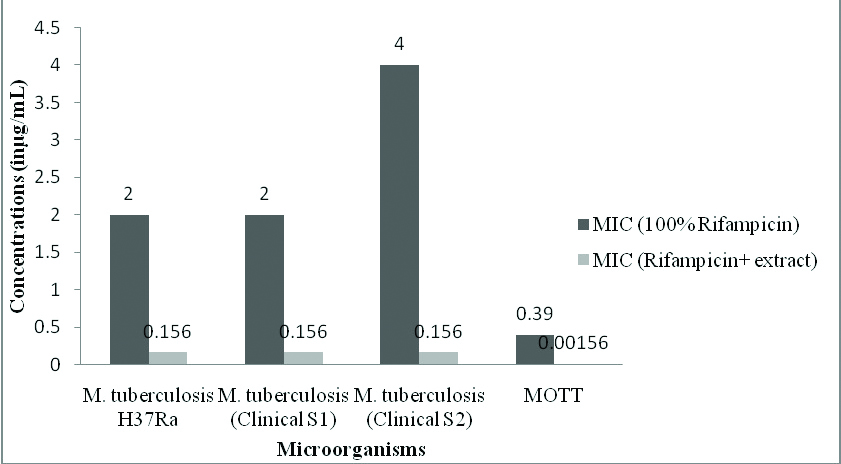
The bar diagram represents the mean of MIC values (in μg/mL) of the rifampicin alone and in combination with the extract of mushroom P. ostreatus.
Discussion
Literature surveys have revealed the presence of derivatives of benzoic acid (protocatechuic acid, vanillic acid, salicylic acid) and cinnamic acid (caffeic acid, p- coumaric acid, ferulic acid) in various species of mushrooms studied earlier [22,23]. In our study we have observed the presence of various such compounds such as p- coumaric acid, epicatechin, sinapic acid, gallic-caffeic acid ester, myricetin rhamnoside, rutin etc., [Table/Fig-5,7,8] which is in accordance with various other literatures reported earlier although their methods of extraction were different from us [17,23]. In another study which was done to analyse the phenol compounds profile of methanolic extracts of eight types of edible mushrooms among which P. ostreatus was one of them revealed the presence of some common compounds such as myricetin, p- coumaric acid [17]. Many of these compounds are well known for their antimicrobial and antioxidant properties as mentioned in different literatures [17,18,23,24]. In this study, we have also observed the presence of flavones apigenin (m/z value 268.1), naringenin-7-o-glucoside/ Chalcone – glucoside (m/z value 435.0) and vitamin B5 residue, i.e., pantothenic acid hexose (m/z value 381.0) [Table/Fig-8] [19-21]. These components are also well known for their antibacterial applications against many common pathogens such as Enterobacter cloaceae, E. aerogenes, Pseudomonas aeruginosa, Micrococcus luteus, Escherichia coli, Bacillus subtilis, Staphylococcus aureus, S. epidermidis, including fungal pathogens such as Aspergillus niger, Candida albicans, Saccharomyces cerevisiae [25,26]. Interestingly no literature was evident regarding the action of these isolated compounds against any Mycobacteria spp. In our study, we observed that the MIC value of the crude contents of the mushroom extracts remain within concentration of 10mg/mL [Table/Fig-3]. The lowered MIC value can be correlated with the LC/MS data which showed the presence of multiple identified compounds such as p- coumaric acid, sinapic acid, gallic-caffeic acid ester, myricetin, apigenin in the extract of this particular mushroom in comparison to the other screened mushrooms in this study. Therefore, it may be due to the synergistic effect of all this bioactive ingredients which resulted in lowering of the MIC value. Many medicinal plants of different countries were explored in respect of their antitubercular activity with variable results [1,3,9]. Few plants could inhibit both international and wild strains of M. tuberculosis to some extent but have no action upon rapid growers like M. fortuitum [1]. Moreover, no haemotoxicity was observed by the ingredients of the crude mushroom extracts, as shown in [Table/Fig-4]. The mushrooms screened were edible in nature thus they are expected not to show any cytotoxicity.
Research on synergistic combinations of plant extracts, its bioactive components and standard drugs against MDR bacteria as it results in various benefits like increased efficiency, with reduced undesirable side effects, obtaining the required therapeutic effect with relatively small doses in comparison when administered alone [27,28]. A study on synergistic interaction between drug isoniazid and extract of Klebsiella vesicatoria against two drug resistant M. tuberculosis strains showed good activity having FICI value of 0.25 and combined concentration of 6.28μg/mL [29]. In our study, we could observe that there is an excellent reduction of MIC value of drug rifampicin followed by ethambutol against the strains of Mycobacteria [Table/Fig-6,9,11]. The drug isoniazid did not show any synergy with the mushroom extract against the strains of M. tuberculosis except MOTT [Table/Fig-10].
Limitation
Further purification of these active components can lead to development of new drug formulation against this deadly disease. Based on the data available from the in vitro study further study in an in vivo model is necessary before any clinical application.
Conclusion
Edible mushrooms can be considered as a potent source of bioactive compounds showing antituberculous activity. As no study is available to the best of our knowledge regarding the antituberculous activity of edible mushrooms, we can consider the present study as a significant contribution to the existing literature.