VanA Mediated Glycopeptide Resistant Enterococcus faecium (GRE) Infection in an Elderly Patient with Chronic Kidney Disease – A Case Report
Martin Jane Esther1, PK Rath2, R Gopalakrishnan3
1 Consultant and HOD, Department of Microbiology, Doctors’ Diagnostic Centre, Trichy, Tamil Nadu, India.
2 Pathologist, Department of Pathology, Doctors’ Diagnostic Centre, Trichy, Tamil Nadu, India.
3 Nephrologist, Department of Nephrology, Maruti Hospital, Trichy, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Martin Jane Esther, C4, 10 B Cross, Thillai Nagar, Trichy-620018, Tamil Nadu, India.
E-mail: janesweety16@gmail.com
A 66-year-old male patient who is a known case of chronic kidney disease was admitted to the hospital with complaint of seizures. He was uremic and showed signs of encephalopathy. He was previously hospitalized frequently and was on antitubercular treatment. A left sided radio cephalic fistula was made and the patient was on dialysis. Urinary catheter and central venous catheter were placed. Urine and blood cultures showed growth of Enterococcus faecium which was resistant to vancomycin – Vancomycin Resistant Enterococci (VRE) or Glycopeptide Resistant Enterococci (GRE) by antibiotic susceptibility testing. The isolate was genotyped for detection of Van A, Van B and Van C genes and was found to harbour Van A gene. The treatment details of the patient and his outcome have been described. As Enterococci are inherently resistant to many drugs, their treatment is quite challenging. Combination therapy and de-escalation from broad spectrum antimicrobials to specific narrow spectrum antibiotics immediately after antibiotic sensitivity report is available which prevents emergence of drug resistance. Spread of VRE infection must be controlled by strict infection control methods as plasmid mediated rapid transfer of VRE can occur to other organisms like Staphylococci which is a serious threat.
Antibiotic susceptibility, Glycopeptide resistant Enterococci, Vancomycin resistant, Enterococcus faecium
Case Report
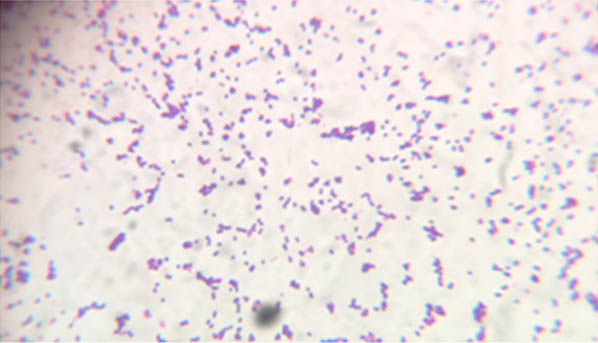
A 66-year-old male patient was admitted to the hospital with complaint of seizures since half an hour. The seizures were sudden in onset and he had no history of seizures in the past. He had no history of fever or headache or vomiting. His general condition was poor and he was extremely malnourished. He had pitting oedema of both the legs up to ankle. His BP was 140/80 mmHg and pulse rate was 82/min. On auscultation of the chest, S1 and S2 were heard normally. He had bronchial breath sounds in the apical region and diminished breath sounds in the basal region of the right lung. Normal vesicular breath sounds were heard in the rest of the lung fields. He was a known case of chronic kidney disease who presented with uraemia and end stage renal disease about 16 weeks earlier, for which he was hospitalized to ICU and haemodialysis was carried out. The patient was discharged with urinary catheter and catheter management was done at home. Then he developed right sided exudative pleural effusion with Elevated Adenosine Deaminase (ADA) and antitubercular treatment was started. A left sided radio-cephalic fistula was made for performing haemodialysis. The patient developed hypotension. So Central Venous Catheter (CVC) was placed in jugular vein for haemodialysis, which was in place for 12 weeks. The patient was then admitted with history of seizures (present admission). Investigations showed uraemia. His blood urea nitrogen and creatinine were 82 and 34 mg/dl respectively. Patient developed signs of encephalopathy and sepsis. On day 2, blood was cultured. Initial three cultures taken two days apart showed no growth. But the general condition of the patient deteriorated, his oxygen saturation decreased for which he was mechanically ventilated on day 10 and started on broad spectrum antibiotics (Meropenem and vancomycin). Initially clinical response of the patient was good. Two days later, patient developed fever, elevated CRP and WBC count. Blood and urine were sent for culture. Urinary catheter was removed and the patient was started on polymyxin B and teicoplanin. Blood culture was positive by Bact Alert in 12 hours. Blood was sub-cultured on mac conkey and blood agar plates and incubated overnight at 37°C. A bacterial isolate which was non haemolytic on blood agar and magenta coloured on mac conkey agar grew in pure culture which showed to be gram positive cocci arranged in pairs with spectacle eyed appearance as shown in [Table/Fig-1], was catalase negative, tolerated 6.5% NaCl, survived for 30 minutes at 60°C, showed blackening of bile esculin agar medium [Table/Fig-2], fermented arabinose and did not ferment sorbitol and pyruvate.
Gram stain of Enterococcus faecium.
a) Enterococcus showing blackening of bile esculin agar slant; b) Uninoculated bile esculin agar slant.

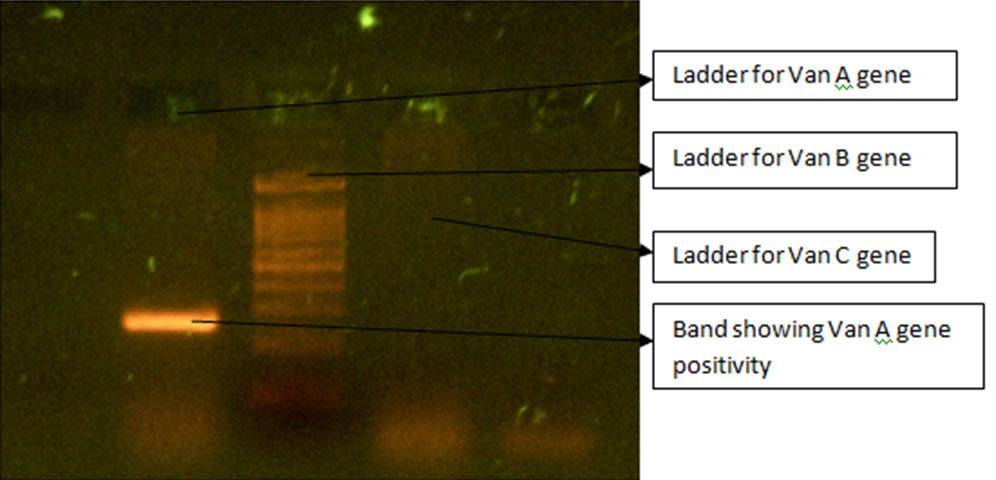
The diagnosis of Enterococcus faecium was made which was confirmed by vitek 2 compact (biomeriux). Urine sample of the patient also grew Enterococcus faecium when processed similarly. Isolates from both the clinical samples showed similar antimicrobial resistance pattern as tested by vitek. The isolate was resistant to penicillin (MIC>64), high level aminoglycosides (HLR), ciprofloxacin (MIC>=8), levofloxacin (MIC>=8), erythromycin (MIC>=8), vancomycin (MIC>=64), teicoplanin (MIC>=64) and nitrofurantoin (MIC=128) (for urinary isolate only) showing to be VRE or GRE according to 2018 CLSI guidelines [1]. The isolate was sensitive to doxycycline, tigecycline (<=0.12), chloramphenicol and linezolid (MIC=1). Thus the isolate proved to be Vancomycin resistant Enterococcus faecium. VRE infection was not anticipated and despite treatment with teicoplanin the patient expired (16th day of admission) due to septic shock on the day the antibiogram was available. The VRE isolate was then genotyped for detection of Van A, Van B and Van C genes by multiplex PCR. The test isolate was found to harbour Van A gene as shown in [Table/Fig-3].
Genotying for Van A, Van B and Van C genes Positive (Band) for Van A gene.
Discussion
Enterococci are gram positive bacteria that normally colonise the gastrointestinal tract of humans and animals. They are facultative anaerobes implicated in a variety of community and hospital acquired infections [2] like septicaemia, endocarditis, urinary tract infections, biliary tract infections and meningitis. Though there are many species of Enterococci, E.faecalis and E.faecium are implicated in majority (90%) of clinical infections [3]. Their intrinsic resistance to commonly used antibiotics, ability to acquire and spread resistance and form biofilms are the properties make Enterococci a significant nosocomial pathogen [4] E.faecalis causes more number of clinical infections (about 80%) than E.faecium. But, mortality is high in E.faecium infections compared to E.faecalis [2]. Bacteraemia due to Enterococci is usually seen in debilitated individuals with underlying disease conditions [5]. Our patient was an elderly individual with chronic kidney disease on haemodialysis. In a study by D’Agata EM et al., VRE is shown to be a common pathogen in patients on chronic haemodialysis which is of great concern. The main mode of transmission from one patient to another has been suggested to be through the hands of healthcare workers [6]. The patient has also suffered from tuberculosis which is an immunosuppressive condition. He was repeatedly hospitalized to ICU which increased the chances of instrumentation, acquiring nosocomial infection and exposure to multiple antibiotics. Repeated hospitalizations and antibiotic exposure increases the risk of colonization with VRE which in turn can predispose to infection [6].
Home management of urinary catheter increased the possibility of community acquired infection. About a third of the patients managing urinary catheter at home have been found to acquire urinary tract infection [7]. As the patient had CVC in place for 12 weeks, there is a possibility that his bacteraemia could have been Central Line Associated Blood Stream Infection (CLABSI). The rate of incidence of CLABSI in those managing CVC at home ranges from 0 to 36 CLABSI per 1,000 CVC-days [8]. The negative initial blood and urine cultures following admission, which later turned positive, indicate that the infection was nosocomial. There is a possibility that it could have been Catheter Associated Urinary Tract Infection (CAUTI) with hematogenous spread. Since the same organism has been isolated from blood and urine, the patient has suffered systemic Enterococcal infection and Enterococcal septicaemia. Initial good clinical response of the patient to vancomycin followed by deterioration suggests that vancomycin resistance might have developed during the course of treatment [8]. Enterococci with Van A gene are resistant to high levels of vancomycin (MIC, 64 mg/mL) and teicoplanin (MIC, 8 mg/mL). Resistance is induced by the presence of either drug. Treatment of this patient with teicoplanin could therefore be the probable reason for this enterococcal isolate to have acquired resistance to both these antibiotics [9]. Vancomycin and teicopalin are glycopeptide antibiotics that act by inhibiting bacterial cell wall synthesis by binding to cell wall precursors. Vancomycin resistance occurs due to Van gene mediated change in the amino acid side chains where the drug has to act. The phenotypes of vancomycin resistance described so far are van A, van B, van C, van D, van E, van G, van L, van M and van N [10]. Van A is the most common phenotype that confers high level resistance (MIC>64) to both vancomycin and teicoplanin and is transferable from one organism to another (acquired resistance) [11]. Our patient was found to be infected with Enterococcus faecium with Van A gene and so he was clinically unresponsive to vancomycin and teicoplanin. Increased mobile genetic elements, hypermutability and metabolic alterations confer drug resistance to E.faecium. About 80% of the E.faecium is resistant to vancomycin as against 5% of Enterococcus fecalis. Enterococci can transfer their resistance genes to other organisms like Staphylococci which is a serious threat. Combination therapy based on sensitivity report is ideal for treatment of VRE infections. Treatment options for VRE include ampicillin (if susceptible) plus an aminoglycoside (If sensitive to high level aminoglycosides). If resistant to aminoglycosides, ampicillin plus ceftriaxone/high dose daptomycin/imipenem (if MIC 64, ampicillin will be ineffective. Combination therapy results in synergistic killing of Enterococci [12]. The main cause for emergence of VRE is the use of vancomycin to treat minor ailments with Enterococci. Vancomycin must be used only when absolutely necessary. When started for empirical treatment, de-escalation to narrow spectrum antibiotics immediately after receiving the antibiotic sensitivity report is necessary. Continuing treatment with broad spectrum antibiotics ends up in the organism acquiring resistance even to the reserve drugs which poses a serious threat to the patient and the physician. The resistant strain can spread both in the hospital and community. So it is necessary to use the right antibiotic for the right patient at the right time. Screening for VRE in patients in ICUs can be done by culture of rectal swabs/stool samples (VRE colonization) or relevant clinical samples based on presenting symptoms (VRE infection) [13].
Patients who are infected or colonized with VRE must be isolated and all contact precautions must be followed while handling the patient. VRE infected individuals must receive specific antibiotic treatment. Strict adherence to infection control practices like hand hygiene, usage of dedicated equipment for patients with VRE, appropriate environmental cleaning, usage of personal protective equipments, proper disposal and handling of biomedical waste can prevent further emergence and spread of drug resistance [14].
Conclusion
Vancomycin resistant Enterococcal infections mainly occur in patients with immunocompromised conditions, repeated or prolonged hospitalizations, mechanical instrumentation and those on prolonged antibiotic therapy. They are a therapeutic challenge to the treating clinician. Therefore better clinical outcomes can be expected if the empirical antibiotic treatment covers vancomycin resistant Enterococci and that too in combination therapy so that emergence of drug resistance can be prevented. It is also absolutely necessary to de-escalate to specific antibiotic regimen immediately after antibiotic susceptibility test results are available which is often not done. Mechanical devices must be placed in patients only when there is an absolute indication. Strict adherence of patients, physicians and other health care workers to infection control measures like hand hygiene, contact and isolation precautions can prevent further spread of drug resistant isolates.
[1]. Performance Standards for Antimicrobial Susceptibility Testing; M100 S28 2018 28th Edition [Google Scholar]
[2]. Ananthanarayan and Paniker; Textbook of microbiology 2017 10th Edition:219-20. [Google Scholar]
[3]. Fraser SL, Donskey CJ, Salata RA, Enterococcal InfectionsMedscape; August 2017 [Google Scholar]
[4]. Miller WR, Munita JM, Ariasn CA, Mechanisms of antibiotic resistance in enterococciExpert Review of Anti-Infective Therapy 2014 12(10):1221-36.10.1586/14787210.2014.95609225199988 [Google Scholar] [CrossRef] [PubMed]
[5]. O’Driscoll T, Crank CW, Vancomycin-resistant enterococcal infections: epidemiology, clinical manifestations, and optimal managementInfection and Drug Resistance 2015 8:217-30.10.2147/IDR.S5412526244026 [Google Scholar] [CrossRef] [PubMed]
[6]. D’Agata EM, Green WK, Schulman G, Li H, Tang YW, Schaffner W, Vancomycin-resistant enterococci among chronic hemodialysis patients: a prospective study of acquisitionClinical Infectious Diseases 2001 32(1):23-29.10.1086/31754911112676 [Google Scholar] [CrossRef] [PubMed]
[7]. Wilde M, McDonald MV, Brasch J, McMahon JM, Fairbanks E, Shah S, Long-term Urinary catheter users self-care practices and problemsJournal of Clinical Nursing 2013 22(0):356-67.10.1111/jocn.1204223301577 [Google Scholar] [CrossRef] [PubMed]
[8]. Keller SC, Williams D, Gavgani M, Hirsch D, Adamovich J, Hohl D, Environmental exposures and the risk of central venous catheter complications and readmissions in home infusion therapy patientsInfection Control and Hospital Epidemiology 2017 38(1):68-75.10.1017/ice.2016.22327697084 [Google Scholar] [CrossRef] [PubMed]
[9]. Gold HS, Vancomycin-resistant enterococci: mechanisms and clinical observations; antimicrobial resistance; CID 2001 July:3310.1086/32181511418881 [Google Scholar] [CrossRef] [PubMed]
[10]. Chen C, Sun J, Guo Y, Lin D, Guo Q, Hu F, High prevalence of vanM in vancomycin-resistant Enterococcus faecium isolates from Shanghai, ChinaAntimicrob Agents Chemother 2015 59(12):7795-98.10.1128/AAC.01732-1526369966 [Google Scholar] [CrossRef] [PubMed]
[11]. Teo JW, Krishnan P, Jureen R, Lin RT, Detection of an Unusual van Genotype in a Vancomycin-Resistant Enterococcus faecium Hospital IsolateJ Clin Microbiol 2011 49(12):4297-98.10.1128/JCM.05524-1121998432 [Google Scholar] [CrossRef] [PubMed]
[12]. Arias CA, Contreras GA, Murray BE, Management of multidrug-resistant enterococcal infectionsClinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases 2010 16(6):1010.1111/j.1469-0691.2010.03214.x20569266 [Google Scholar] [CrossRef] [PubMed]
[13]. d’Azevedo PA, Santiago KA, Furtado GH, Xavier DB, Pignatari AC, Titze-de-Almeida R, Rapid detection of Vancomycin-Resistant Enterococci (VRE) in rectal samples from patients admitted to intensive care unitsBraz J Infect Dis 2009 13(4):289-93.10.1590/S1413-8670200900040001020231993 [Google Scholar] [CrossRef] [PubMed]
[14]. Mutters NT, Mersch-Sundermann V, Mutters R, Brandt C, Schneider-Brachert W, Frank U, Control of the spread of vancomycin-resistant enterococci in hospitals: epidemiology and clinical relevanceDtsch Arztebl Int 2013 110(43):725-31.10.3238/arztebl.2013.072524222791 [Google Scholar] [CrossRef] [PubMed]