Acute Hypersensitivity Reaction Related to use of Pantoprazole Lacking Cross-Reactivity with Omeprazole
Hemangini Rajanikant Acharya1, Manish Jasmatbhai Barvaliya2, Tejas Kamleshbhai Patel3, C B Tripathi4
1 Tutor, Department of Pharmacology, Government Medical College Bhavnagar, Gujarat, India.
2 Assistant Professor, Department of Pharmacology, Government Medical College Bhavnagar, Gujarat, India.
3 Assistant Professor, Department of Pharmacology, GMERS Medical College, Gujarat, India.
4 Dean & Professor, Department of Pharmacology, Government Medical College Bhavnagar, Gujarat, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Manish Jasmatbhai Barvaliya Department of Pharmacology, Government Medical College, Near ST Bus Stand, Jail Road, Bhavnagar, Gujarat, India
E-mail: drmanishbarvaliya@gmail.com
Proton Pump Inhibitors (PPIs) are widely used drugs for peptic ulcer and other acid related disorders those are rarely causing allergic reactions. Cross-reactivity between the various PPIs has been documented. In present case, a 57-year-old male patient developed urticaria and itching all over the body after 30 minutes of taking second dose of pantoprazole. He also had eye congestion, oedema below the eyes and over nose with some skin exfoliation over forehead. Urticaria and itching relieved within one hour with the use of levocetrizine. Other symptoms recovered within 48 hours. Further doses of pantoprazole were avoided and patient took two doses of omeprazole that did not cause any allergic reaction. Even though, patient did not show cross-reactivity between pantoprazole and omeprazole, use of other PPIs should be avoided when patient is hypersensitive to one PPI as it is difficult to judge cross-reactivity clinically.
Adverse drug reaction, Allergic reaction, Cross-reactivity, Omeprazole, Pantoprazole, Proton pump inhibitors, urticaria
Case Report
A 57-year-old male patient (weight 65 kg) came to the medical out-patient department of a tertiary care hospital, Bhavnagar, Gujarat, India with complaints of nausea and epigastric burning pain for last 2 days where he was prescribed tablet Pantoprazole 40 mg orally 12 hourly and tablet Domperidone 10 mg as and when required. He was a known case of schizophrenia and on tablet Olanzapine 2.5 mg at bed time since last six years. The patient took the first dose of tablet Pantoprazole 40 mg and tablet Domperidone 10 mg at bedtime with his regular medicine. Next day morning, the patient took pantoprazole on an empty stomach and did not take Domperidone as the nausea was relieved. Within 30 minutes of taking Pantoprazole, the patient developed urticaria with itching all over the body. He also developed oedema and redness below the eyes and over the nose, with mild congestion in both eyes and exfoliation of skin on forehead [Table/Fig-1].
Sign and symptoms of hypersensitivity reaction with pantoprazole after 24 hours of occurrence of event.
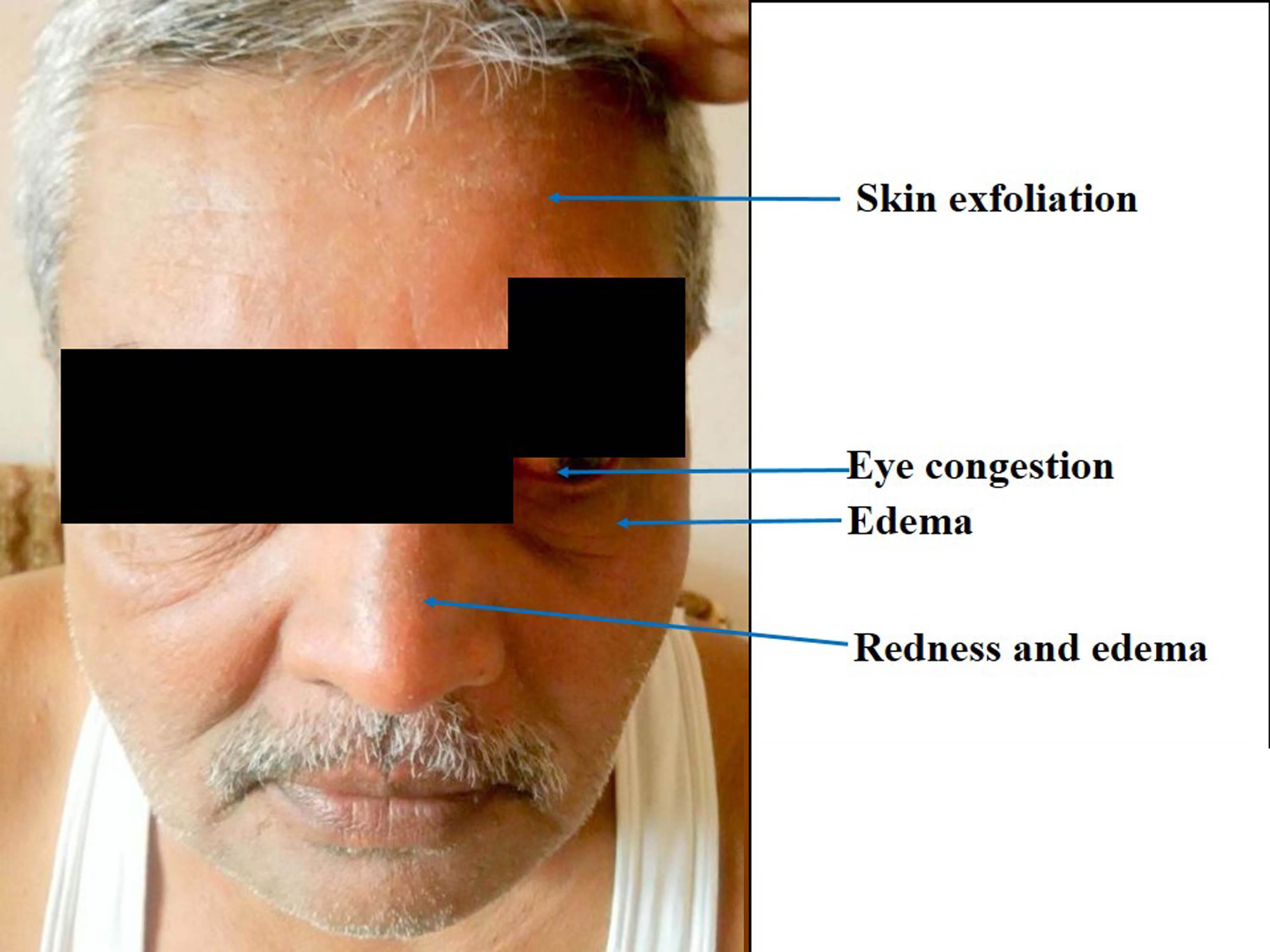
The patient took tablet levocetrizine 5 mg immediately. He recovered from urticaria and itching within 1 hour. Other reactions like oedema, redness and exfoliation of skin recovered gradually within 48 hours. The patient did not take further doses of Pantoprazole. On detailed drug history, the patient was using on and off Omeprazole for gastritis. He was asked to switch over to capsule Omeprazole 20 mg based on his uneventful history. The patient took two doses of omeprazole without any event (Oral challenge). On detailed history, the patient revealed that he had some episodes of urticaria before and the last episode was before the 4 years. Previous episodes of urticaria were associated with intake of some citrus fruits and pickles, avoidance of which resulted in the relief from urticaria. They were never associated with the drug. The patient did not have any other history of drug allergy. The patient did not take pantoprazole or any other PPIs except omeprazole ever before this event. The event was probably related to pantoprazole when assessed with Naranjo algorithm scale (score 6) [1].
Discussion
PPIs are commonly used drugs for peptic ulcer and other acid related disorders. Omeprazole is a prototype, while Lansoprazole, Rabeprazole, Pantoprazole, and Esomeprazole developed later. PPIs are generally well tolerated and considered causing minimal adverse effects [2]. Their common adverse effects are nausea, loose stools, headache, dizziness, abdominal pain, muscle and joint pain. The literature suggests that rashes, urticaria, angiooedema and other allergic reactions are rarely associated with the PPIs [3-5]. Cross reactivity among different PPIs is also documented [4,5]. Earlier reports suggest that omeprazole share the cross reactivity with pantoprazole [4,5]. However, we observed a case of acute hypersensitivity reaction due to Pantoprazole lacking the cross reactivity to Omeprazole. The present adverse event showed a positive temporal relationship with Pantoprazole. The patient took the second dose in the morning with empty stomach that excluded the food related urticaria. In this case, the patient was naïve to pantoprazole and he developed the reaction immediately after the second exposure to Pantoprazole that indicates an IgE mediated mechanism [4,5].
Sobrevia Elfau MT et al., evaluated the cross-reactivity between Omeprazole, Pantoprazole, Esomeprazole, Rabeprazole, and Lansoprazole through the Skin Prick Test (SPT) in 5 known cases of Omeprazole related immediate hypersensitivity reactions [5]. On SPT, the authors observed all possible results: (a) hypersensitive to one and cross-reactivity with all PPIs; (b) cross-reactivity between a particular pair of drugs (Omeprazole and Pantoprazole; Lansoprazole and Rabeprazole) while tolerability to others; (c) hypersensitivity to one but tolerability to all other PPIs [5]. It suggests that hypersensitivity reaction to PPIs can be due to common core structure or different substitutions. Here in this case, lack of cross reactivity to Omeprazole was confirmed through the oral challenge test. However, we could not check cross-reactivity to all other PPIs due to ethical reason.
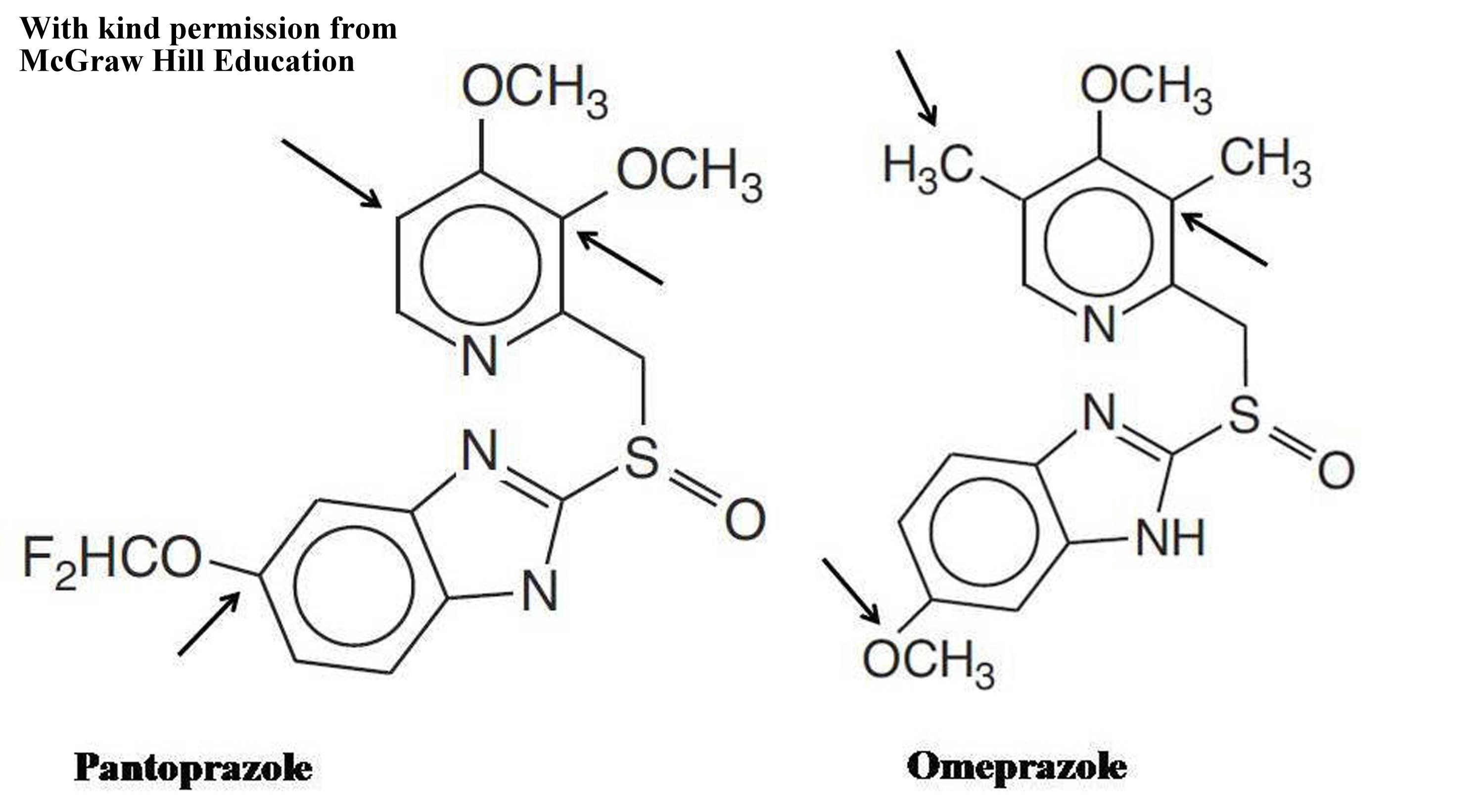
All the PPIs have a common core structure that is 2-pyridylmethylsulfinylbenzimidazole. Pattern of hypersensitive to one and cross-reactivity with all PPIs may be due to allergic reaction to core structure as seen with flouroquinolones [6]. However, pattern of cross-reactivity between a particular pair and hypersensitivity to one and tolerability to all other PPIs might be due to differences in substitution on pyridine and bezimidazole rings [5,7]. Omeprazole and Pantoprazole differ in their substitutions on benzimidazole ring with methoxy and difluoromethoxy chain, respectively [Table/Fig-2] [5,8]. Whereas, pyridine ring contains two methyl-one methoxy group and two methoxy groups in Omeprazole and Pantoprazole, respectively [Table/Fig-2] [5,8]. Even though, being a common pair of cross-reactivity, lacking cross-reactivity between Pantoprazole and Omeprazole was noted in present case. It suggests that our patient may have tolerability to all other PPIs. However, it is very difficult to judge clinically that patient who develops hypersensitivity to one PPI will follow which type of cross-reactivity pattern with other PPIs.
Chemical structure differences for pantoprazole and omeprazole.
Arrows indicates the positions of structural differences.
Conclusion
Use of other PPIs should be avoided when patient is hypersensitive to one PPI without confirming cross-reactivity using skin prick test or intra-dermal sensitivity test. Patient should be shifted to H2 blockers to avoid the risk of cross-reactivity of PPIs.
In our institute, Institutional Review Board (IRB), Government Medical College, Bhavnagar (Regd. No.ECR/557/Inst/GJ/2014, Dated: 29/04/ 2014, Regd. with: Directorate General of Health Services Office of Drugs Controller General of India) does not require approval to report a case of adverse drug reaction.
Informed consent was taken from the patient.
Financial or Other Competing Interests
None.
Informed Consent was Obtained from the Patient for Publishing the Case Report.
[1]. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, A method for estimating the probability of adverse drug reactionsClin Pharmacol Ther 1981 30(1):239-45.10.1038/clpt.1981.1547249508 [Google Scholar] [CrossRef] [PubMed]
[2]. Yu LY, Sun LN, Zhang XH, Li YQ, Yu L, Meng L, A review of the novel application and potential adverse effects of proton pump inhibitorsAdvances in Therapy 2017 34(5):1070-86.10.1007/s12325-017-0532-928429247 [Google Scholar] [CrossRef] [PubMed]
[3]. Chang YS, Hypersensitivity reactions to proton pump inhibitorsCurr Opin Allergy Clin Immunol 2012 12:348-53.10.1097/ACI.0b013e328355b8d322744268 [Google Scholar] [CrossRef] [PubMed]
[4]. Lobera T, Navarro B, Del Pozo MD, González I, Blasco A, Escudero R, Nine cases of omeprazole allergy:cross-reactivity between proton pump inhibitorsJ Investig Allergol Clin Immunol 2009 19:57-60. [Google Scholar]
[5]. Sobrevia Elfau MT, Garcés Sotillos M, Ferrer Clavería L, Segura Arazuri N, Monzón Ballarin S, Colás Sanz C, Study of cross- reactivity between proton pump inhibitorsJ Investig Allergol Clin Immunol 2010 20:157-61. [Google Scholar]
[6]. Anovadiya AP, Barvaliya MJ, Patel TK, Tripathi CB, Cross sensitivity between ciprofloxacin and levofloxacin for an immediate hypersensitivity reactionJ Pharmacol Pharmacother 2011 2:187-88.10.4103/0976-500X.8328521897714 [Google Scholar] [CrossRef] [PubMed]
[7]. Anderson S, Christensen S, Hypersensitivity reaction to omeprazole in a patient treated for Helicobacter pyloriTherap Adv Gastroenterol 2015 8(2):101-04.10.1177/1756283X1456431425729434 [Google Scholar] [CrossRef] [PubMed]
[8]. Wallace JL, Sharkey KA, Pharmacotherapy for Gastric Acidity, Peptic Ulcers, and Gastroesophageal Reflux Disease. In: Brunton LL. Gilman AG Editors. Goodman and Gilman’s The pharmacological Basis of Therapeutics 1996 12th EditionNew YorkMcGraw-Hill:1311 [Google Scholar]