Mycobacterium fortuitum Bacteraemia in a Immunocompromised Patient of Invasive Ductal Carcinoma of Breast and Long Term Venous Access Device
Swati Kumari1, Kopula Sathyamurthy Sridharan2, R Packia Nancy3, Gifty Sara Mathew4
1 Senior Resident, Department of Microbiology, Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India.
2 Professor, Department of Microbiology, Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India.
3 Post Graduate Student, Department of Microbiology, Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India.
4 Post Graduate Student, Department of Microbiology, Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Kopula Satyamurthy Shidharan, Professor, Department of Microbiology, Sri Ramachandra Medical College and Research Institute, Chennai-600116, Tamil Nadu, India.
E-mail: sridharshyama@gmail.com
Mycobacterium fortuitum is rapidly growing mycobacteria ubiquitous in nature. We report a case of Mycobacterium fortuitum bacteraemia in a immunocompromised 62-year-old female patient of carcinoma breast who underwent mastectomy one year back. Patient was on intensive chemotherapy for past six months and having in situ intra vascular device. Patient was admitted with complaint of fever on and off for which multiple blood samples were collected from patient for three consecutive days from both peripheral and central venous catheter which was subjected to BACTEC® automation system followed by direct microscopic examination and culture. The identification to species level was performed by phenotypic, biochemical and DNA sequencing targeting heat shock protein 65 (hsp 65) gene. Patient improved remarkably on removal of catheter and receiving appropriate antimicrobial therapy.
Central venous catheter, hsp 65 gene, Rapidly growing mycobacteria
Case Report
A 62-year-old lady came with complain of high grade fever of four days duration who had been diagnosed with invasive ductal carcinoma of a breast and had undergone prophylactic bilateral mastectomy a year back. Following surgery, chemotherapy was initiated. Following completion of five cycle of chemotherapy patient developed high grade fever. The cardio vascular system, central nervous system, respiratory system on examination were normal. There was no evidence of skin and soft tissue infection. There was indwelling in-situ port for chemotherapy placed five months back.
Laboratory investigations showed neutropenia, anemia and thrombocytopenia. SGOT and SGPT showed mild elevation 60U/L and 63U/L respectively. Renal function test was within normal limit. Ultrasound abdomen, x-ray chest and paranasal sinuses were normal.
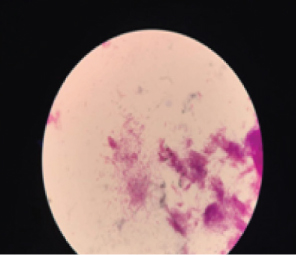
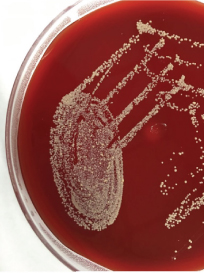
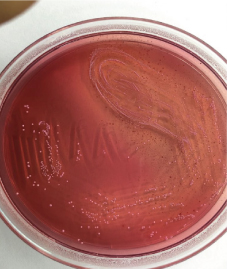
Multiple blood samples were collected from patient for three consecutive days from both peripheral and central venous devices after 72 hours of hospitalization. Blood culture was carried out in BACTEC® automation system, machine gave positive signal after 48 hours following that smears were made from broth [Table/Fig-1]. Broth smear showed slender, curved occasionally branching gram-positive bacilli which was differentiated from diphtheroid by Albert staining. Subsequent acid-fast staining showed uniformly stained acid-fast bacilli [Table/Fig-2]. Routine subculture was done on blood agar, MacConkey agar and the plates were incubated at 37°C for 48hours. Since gram stain showed occasional branching, sample was also sub-cultured on two Lowenstein Jensen (LJ) medium, one LJ medium was incubated at 37°C and other was incubated at 25°C for one week. Blood agar plate showed dry white-yellow colonies [Table/Fig-3], MacConkey agar revealed pink colonies after 48hours of incubation [Table/Fig-4] and Lowenstein-Jensen medium showed non-pigmented colony after 72 hours of incubation [Table/Fig-5].
Photomicrograph of gram stain from BACTEC blood culture bottle showing gram positive bacilli.100 x view.
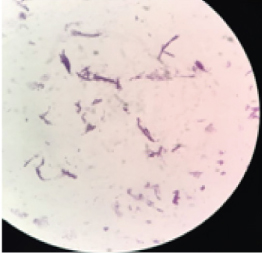
Photomicrograph of modified acid-fast staining from BACTEC blood culture bottle showing acid fast bacilli.100x view.
Showing dry, small, off -white colonies on blood agar after 48 hours of incubation.
Showing small, pink colonies on MacConkey agar after 48 hours of incubation.
Showing non- pigmented, rough, dry colonies on Lowenstein-Jensen medium after four days of incubation.

Smear of colonies from blood agar revealed gram positive bacilli. Colonies were subjected to acid fast staining which showed regularly stained acid-fast bacilli. It was identified as M.fortuitum based on their rapid growth at 25°C and 37°C with no pigment production, positive nitrate reduction, 5% salt tolerance test, and polymyxin B(300 units) disc diffusion test showing zone of inhibition ≥ 10. Isolates of the M.fortuitum group exhibit a clear zone of inhibition (≥10 mm) around polymyxin disc, which drove us towards the diagnosis of M.fortuitum whereas other rapidly growing mycobacteria shows no zone of inhibition for Polymyxin [1].
The antibiotics used were amikacin, cefoxitin, ciprofloxacin, linezolid, imipenem, cotrimoxazole, doxycycline for testing as per CLSI 2017 guideline, and susceptibility testing was performed by Kirby- Bauer disc diffusion method as per literature evidence, by using commercially available discs proclaimed by Hi-Media [1]. The isolates were in- vitro susceptible to amikacin, ciprofloxacin, imipenem, linezolid and resistant to doxycycline, cotrimoxazole. Following that patient was put on linezolid. The catheter was removed and subjected for semi quantitative culture which also yielded growth M.fortuitum. The isolates were confirmed as M.fortuitum by real-time PCR using primer PSL (forward 5’- AGG ATT AGA TAC CCT GGT AGT CCA-3’ and P13P (reverse 5’- AGG CCC GGG AAC GTA TTC AC-3’, based DNA sequencing targeting hsp 65 gene. The patient’s fever subsided after four days of initiation of antimicrobial therapy, linezolid 600 mg twice a day and removal of intravascular catheter. Patient got discharged after a week of antimicrobial therapy and was advised to continue for next three weeks. After discharge, patient has not come for follow up. These finding correlate with pathogenicity of Rapidly Growing Mycobacteria (RGM) species to form a microbial biofilm matrix on the surface of indwelling catheters.
Discussion
Mycobacterium is a slightly curved bacillus that sometimes can show branching filamentous forms like a fungus. Mycobacteria other than mammalian tubercle bacilli which can rarely cause human disease are named Non-Tuberculous Mycobacteria (NTM). Runyoun’s classified NTM into four groups based on pigment production and rate of growth, group IV includes RGM, which can form visible colonies within seven days of subculture on Lowenstein- Jensen media. M.fortuitum, M.chelonae and M.abscesses being the most commonly isolated human pathogens in this group [1]. These atypical mycobacteria are ubiquitous in environment and contaminates water sources and are relatively resistant to routinely used sterilizers and disinfectant [2]. These RGM can lead to wide range of clinical symptoms such as pulmonary infections, skin and soft tissue infections, osteomyelitis, catheter related bacteremia, endocarditis, peritonitis, endophthalmitis in both immunocompetent and immunocompromised host [1]. They are mostly due to accidental inoculation following injection (intramuscular/intravascular), surgery or trauma. Their ability to form a biofilm is a responsible factor in catheter related blood stream infection which is a commonest form of health care associated infection caused by RGM [3].
M.fortuitum shows low inherent pathogenicity but when gets accidental inoculation into sterile body site, can lead to serious infections which is refractile to treatment. Nosocomial infection can also occur from the material used for surgical procedures and it becomes difficult to trace the source of infection in sporadic cases because patients develop clinical symptoms after several days to months of procedure, as seen in our case [4].
In previous 36 studies including 151 patients with cancer and catheter related blood stream infection, the most commonly isolated species was M.mucogenicum 46 (30%) followed by M.fortuitum 34(22%) [5]. There has been few published report of M.fortuitum septicemia in carcinoma of breast patient receiving long term chemotherapy.
Optimal treatment for rapidly growing mycobacteria is catheter removal and minimum of 4 to 8 weeks of antimicrobial therapy which was given in our case also [6].
As per latest report expression of inducible 23S rRNA erm(39) gene in M.fortuitum conveys macrolide resistant which brings concern of treatment with macrolide monotherapy [7].
hsp 65 gene shows more polymorphism than 16S rRNA gene sequence, so it is prospectively more useful for identification of genetically related mycobacterial species [7].
Conclusion
Awareness of possible infection by RGM is important, especially when there are underlying comorbid conditions like immunocompromised, on chemotherapy.
These goes under reported in laboratories because many times gram positive bacilli from blood may be dismissed as diphtheroid and not proceeded for further identification. So, high degree of clinical suspicion and appropriate microbiological techniques are necessary to avoid delay in diagnosis.
[1]. García-Agudo L, García-Martos P, Clinical significance and antimicrobial susceptibility of rapidly growing mycobacteriaScience against Microbial Pathogens: Communicating Current Research and Technological Advances 2011 :77 [Google Scholar]
[2]. Kannaiyan K, Ragunathan L, Sakthivel S, Sasidar AR, Surgical site infections due to rapidly growing mycobacteria in puducherry, IndiaJournal of Clinical and Diagnostic Research: JCDR 2015 9:DC0510.7860/JCDR/2015/10572.563825954616 [Google Scholar] [CrossRef] [PubMed]
[3]. El Helou G, Viola GM, Hachem R, Han XY, Raad II, Rapidly growing mycobacterial bloodstream infectionsThe Lancet Infectious Diseases 2013 13(2):166-74.10.1016/S1473-3099(12)70316-X [Google Scholar] [CrossRef]
[4]. Suseela KV, Ashok ON, Sathiavathy KA, Mycobacterium fortuitum infection following inguinal hernia repair with mesh: A case seriesJournal of the Academy of Clinical Microbiologists 2013 15(2):6910.4103/0972-1282.124592 [Google Scholar] [CrossRef]
[5]. Snydman DR, Redelman-Sidi G, Sepkowitz KA, Rapidly growing Mycobacteria infection in patients with cancerClinical Infectious Diseases 2010 51(4):422-34.10.1086/65514020617902 [Google Scholar] [CrossRef] [PubMed]
[6]. Apiwattankul N, Flynn PM, Hayden RT, Adderson EE, Infections caused by rapidly growing Mycobacteria spp in children and adolescents with cancerJ Pediatric Infect Dis Soc 2014 4(2):104-13.10.1093/jpids/piu03826407409 [Google Scholar] [CrossRef] [PubMed]
[7]. Nash KA, Zhang Y, Brown-Elliott BA, Wallace Jr RJ, Molecular basis of intrinsic macrolide resistance in clinical isolates of Mycobacterium fortuitumJournal of Antimicrobial Chemotherapy 2005 55(2):170-77.10.1093/jac/dkh52315590712 [Google Scholar] [CrossRef] [PubMed]