The majority of Endometrial Carcinomas (EC) is type 1 endometrioid type followed by rare type 2 serous papillary type. Serous papillary EC is a rare but highly aggressive form of uterine endometrial cancer. This is a case of a postmenopausal 57-year-old patient who presented with abnormal uterine bleeding without a specific medical history. Endometrial curettings were taken followed by total abdominal hysterectomy. Histopathology showed serous papillary EC associated with rare features such as unusual site and not invading endocervical mucosa, with accompanying myometritis and senile cystic atrophy of endometrium.
Myometritis, Post menopausal bleeding, Senile cystic endometrial atrophy
Case Report
A 57-year-old, sterilised, post-menopausal female (two para and one live child) presented with copious vaginal bleeding. There was no previous history of abnormal uterine bleeding. Per vaginal and per speculum examination revealed blood clots in the vagina. Transvaginal ultrasound found no abnormalities in the uterus or ovaries. Endometrial thickness was normal. Magnetic Resonance Imaging (MRI) detected haematometra and no endometrial polyp was identified.
Endometrial curettings were sent for histopathological examination which was reported as serous papillary EC type 2. Following this abdominal hysterectomy was done and uterus with cervix and bilateral adnexa were sent along with right paracolic gutter peritoneum, right pelvic peritoneum with right pelvic lymph nodes, left paracolic gutter peritoneum and omentum.
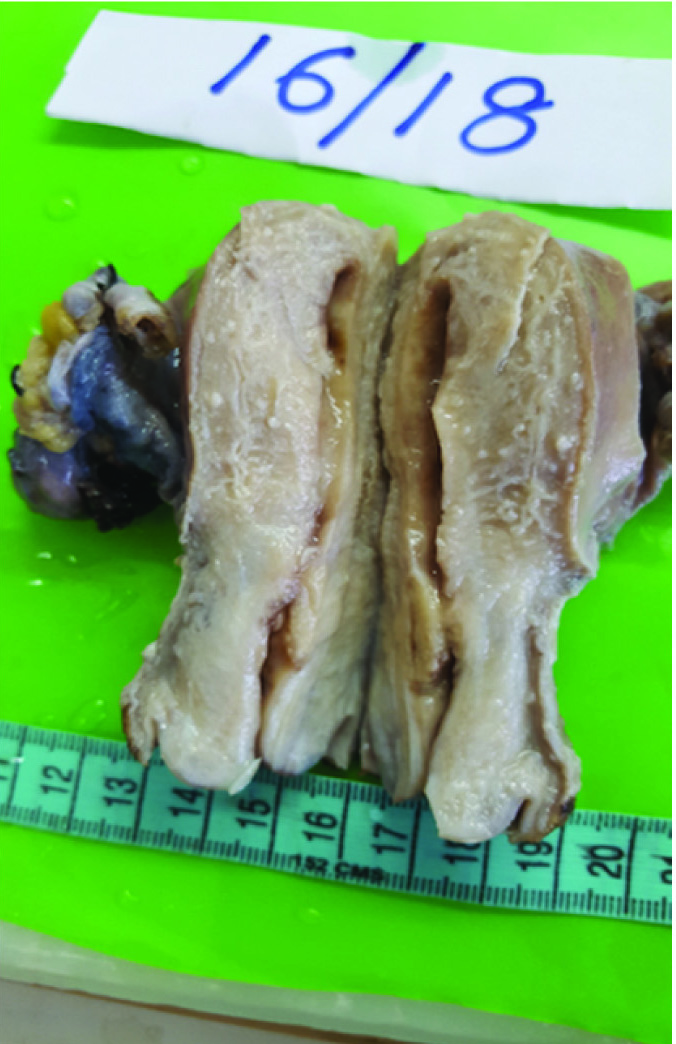
Grossly partly cut open uterus with cervix measuring 8.5×4×4 cm was received. As shown in [Table/Fig-1] cut-section of uterus revealed greyish white tumour at the junction of endocervix and endometrium measuring 1.5×1.5×1.5 cm. Endometrial canal measured 4cm, endometrial thickness was 0.2 cm and endomyometrial thickness was 2 cm on both sides. Cut section of cervix was unremarkable and endocervical canal measured 1 cm. Bilateral adnexa, right paracolic gutter peritoneum, right pelvic peritoneum with right pelvic lymph nodes, left paracolic gutter peritoneum and omentum were unremarkable grossly.
Gross appearance. Uterus with cervix appeared small and atrophic with a small infiltrative growth in lower uterine segment.
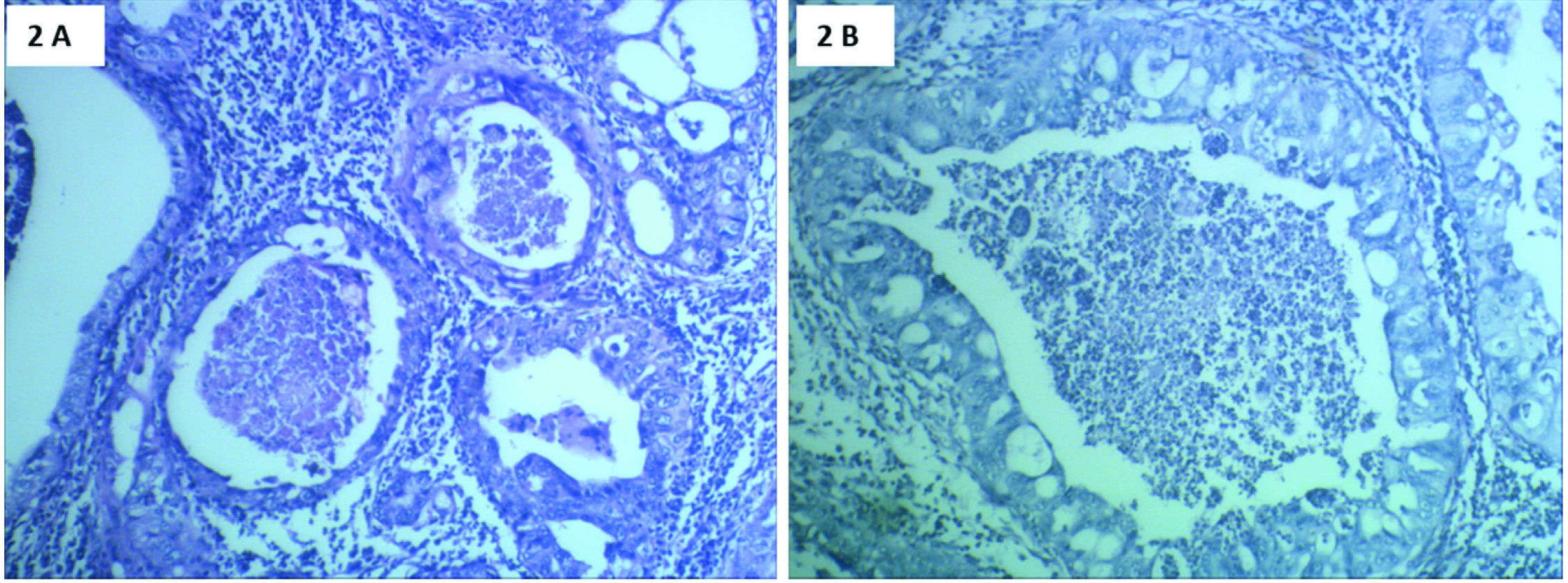
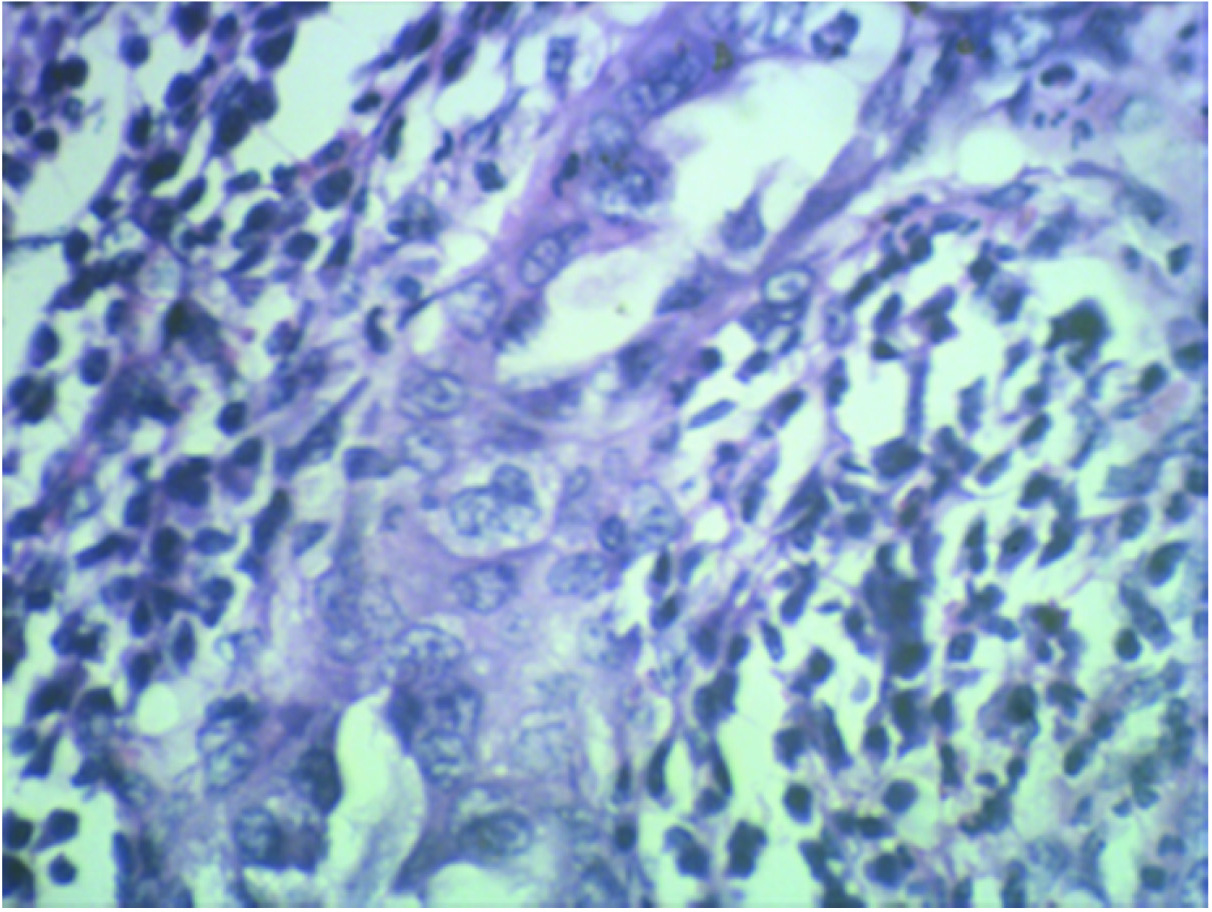
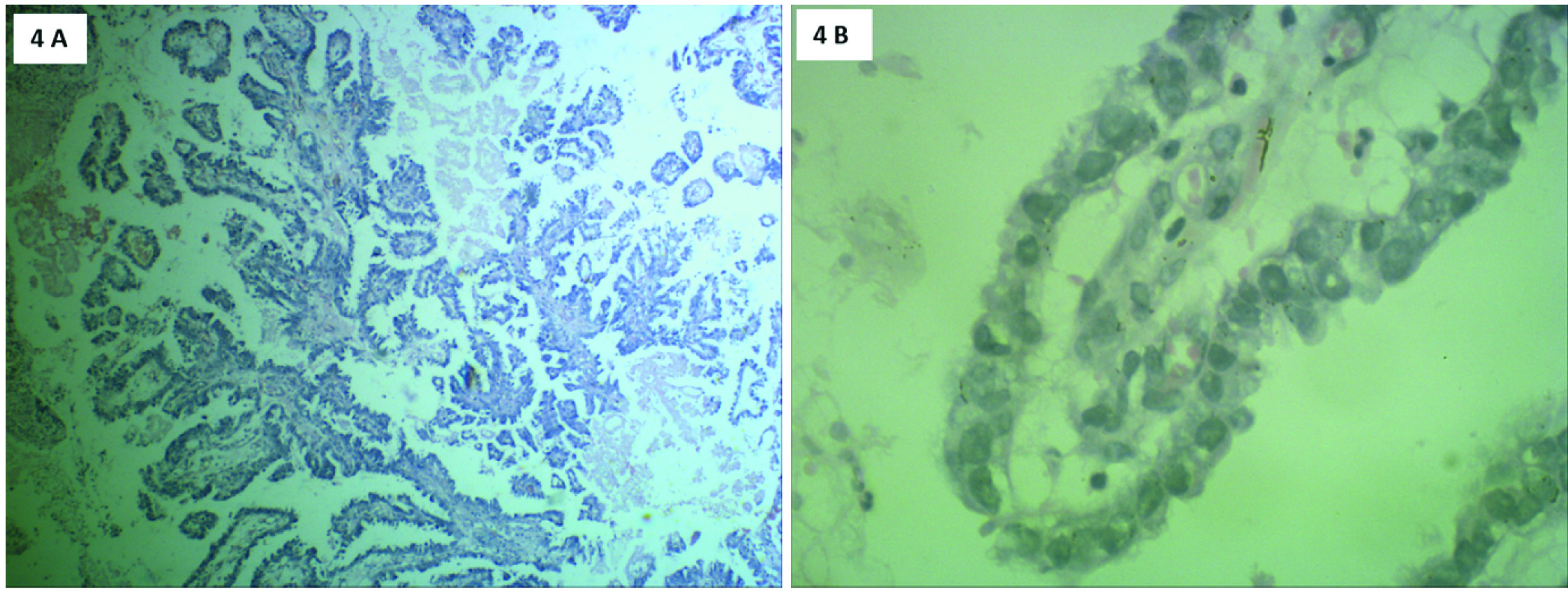
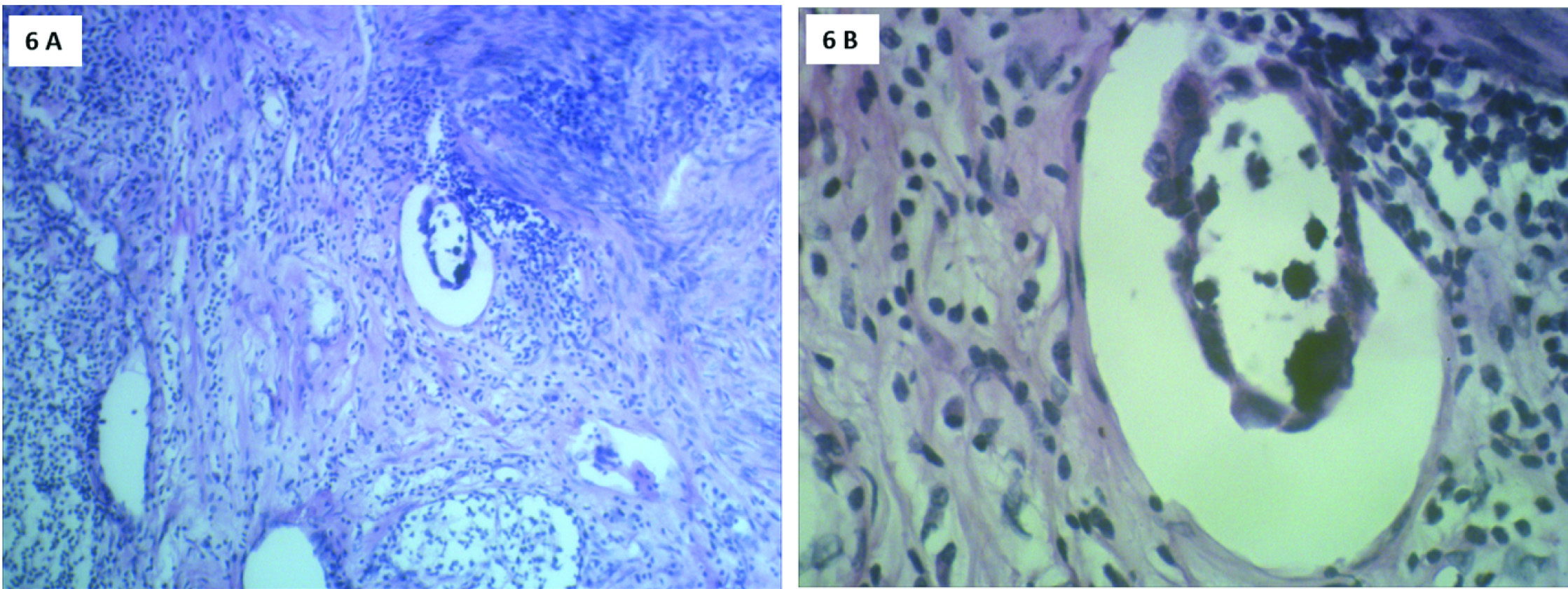
Microscopically, sections from endometrial tumour showed a pattern of serous papillary carcinoma with tumour arranged in glandular pattern [Table/Fig-2] and intraglandular papillary foldings. There was extensive comedo pattern of necrosis in the central portion of the glands [Table/Fig-2]. Focal areas showed squamous metaplasia [Table/Fig-3] and mucin secretion. Areas showing papillary pattern were also seen [Table/Fig-4]. Tumour cells showed multilayering, hobnailing, pleomorphism and hyperchromatism [Table/Fig-5]. The tumour extended irregularly into the adjoining myometrium to a variable depth of one third but not exceeding 50% in one focus. Surrounding the glands and focally in the rest of myometrium were dense lymphoid aggregates [Table/Fig-6]. Parts of the residual uninvolved endometrium from the upper uterine segment showed senile cystic pattern. Sections from cervix showed chronic cervicitis with ulceration and squamous metaplasia. EC extended upto endocervical region but did not involve the mucosa. One right pelvic lymph node was involved by the tumour whereas bilateral adnexa, right paracolic gutter peritoneum, right pelvic peritoneum, left paracolic gutter peritoneum and omentum were uninvolved.
H&E stain. Tumour cells in glandular pattern showing comedo necrosis. 2a) Low power view (10X magnification); 2b) High power view (40X magnification).
H&E stain: High power view (40X magnification). Tumour cells showing squamous differentiation.
H&E stain: Tumour cells arranged in papillary pattern. 4a) Scanner view (4X magnification); 4b) High power view (40X magnification).
H&E stain: High power view (40X magnification). Tumour cells showing hobnailing, focal cytoplasmic clearing, nuclear pleomorphism and high N:C ratio.
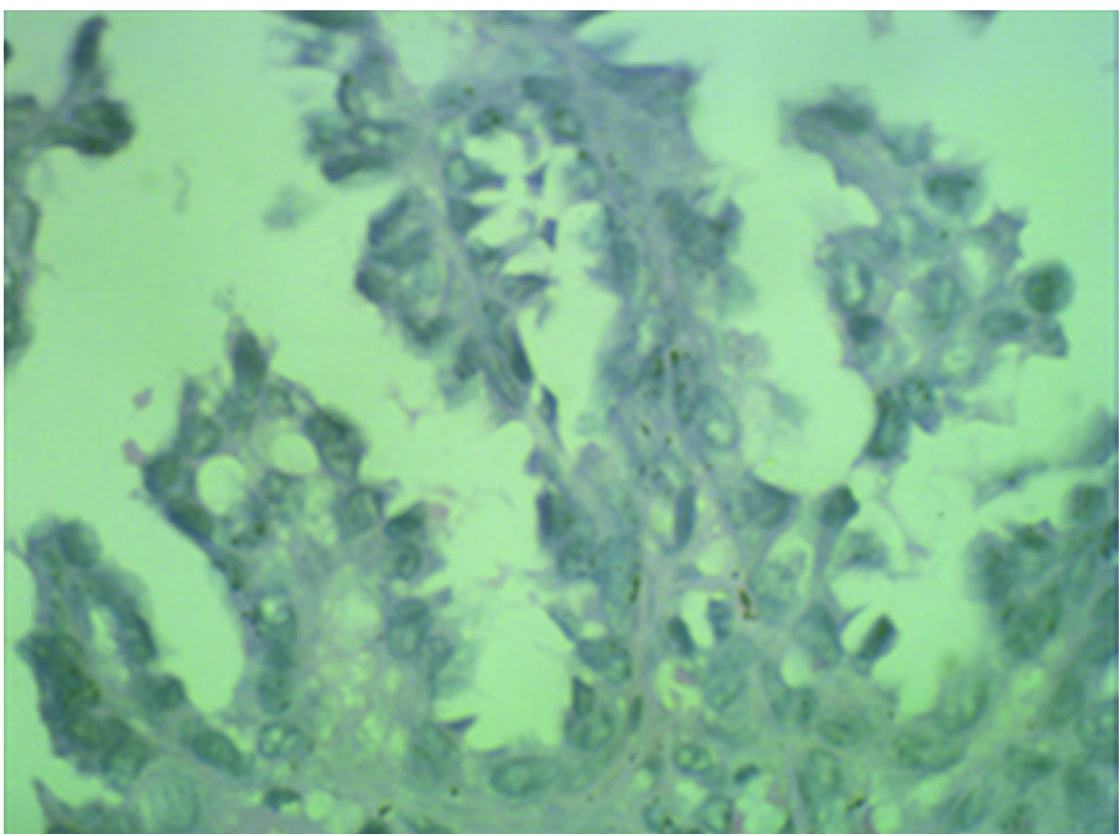
H&E stain: 6a) Scanner view (4X magnification) Adjoining myometrium showing lymphocytic infiltration. 6b) High power view (40X magnification). Lymphatic invasion of tumour cells.
So it was diagnosed as EC, type 2, moderately differentiated, serous papillary type with focal adenosquamous differentiation, involving the inner one-third of myometrium and one pelvic lymph node.
Adjuvant chemotherapy with doxorubicin, paclitaxel and carboplatin were given. Six cycles were administered postoperatively at 3 week intervals. Follow up after 9 months of surgery detected no sign of recurrence.
Discussion
The majority of EC are type 1 Endometrioid Endometrial Carcinoma (EEC), they are oestrogen-related low-grade carcinomas presenting clinically at early-stage, having a 5-year survival rate of greater than 85% [1]. The second type 2 EC are not related to oestrogen and lead to most of the mortality caused by EC. Type 2 EC even when confined to the endometrium can lead to tumour recurrence and death [2].
Serous Carcinoma (SC) comprises most of the type 2 EC and 10% of all EC [3]. It is a very aggressive tumour usually arising in endometrial polyps and occasionally from precancerous lesions developing in atrophic endometrium that mainly occur in older women [4]. The 2Serous Endometrial Intraepithelial Carcinoma (SEIC) is limited to the uterine epithelium, but has been shown to be the precursor of invasive SC [2]. In this case there was unusual association of SC with atrophic endometrium.
WHO 2014 defines a tumour having complex papillary and/or glandular arrangements, with diffuse marked nuclear pleomorphism as SC [5].
The gross appearance of SC and EEC are similar. The uterus is usually enlarged, but occasionally small and atrophic. Tumours are large, bulky, and single or multi nodular protruding into the endometrial cavity, associated with necrosis frequently. Usually there is deep myometrial invasion. Sometimes the tumour is restricted to the lining of an endometrial polyp [6]. Herein as shown in [Table/Fig-1,2] the uterus was small and atrophic, with small infiltrative growth in the lower uterine segment, extended irregularly into the adjoining myometrium.
SC usually has a prominent papillary pattern showing thick, complex, edematous, or fibrotic papillae may be associated with cellular budding, micro- papillary formation, hobnailing, and pseudostratification of tumour cells [7,8]. Tumour cells are pleomorphic and cuboidal having abundant eosinophilic cytoplasm along with focal areas of clear cells may be seen. The nuclei are hyper- chromatic or vesicular with prominent macronucleoli. In some areas the tumour may show a solid pattern or a glandular pattern. Psammoma bodies may also be seen. Often there is deep myometrial invasion and extensive lymphatic invasion. Myometrial invasion shows a ‘gaping gland’ appearance usually not accompanied by stromal reaction. Non-invasive tumour cells may replace endometrial surface or glandular epithelium, without invading the myometrium [6]. In this case we had similar serous papillary pattern associated with chronic myometritis and myometrial invasion. The tumour extended upto endocervical region but did not involve the mucosa.
It has to be differentiated from high grade EEC. True glandular differentiation, along with squamous, mucinous, or secretory change favours EEC. Adjacent complex endometrial hyperplasia/endometrioid intraepithelial neoplasia favours an EEC, whereas the presence of Endometrial Intraepithelial Carcinoma (EIC) supports the diagnosis of SC [9-12]. Herein we had an adenosquamous component which is unusual in SC but all other features supported the diagnosis of SC with a papillary pattern.
Differentiation from low grade EEC is a very important [13], as some SC show a prominent glandular pattern, mimicking a low grade EEC at low power magnification [10-12]. Presence of microcytic pattern, along with squamous, mucinous, or secretory change favours EEC. In contrast, SC shows marked pseudostratification, true luminal border and lack of nuclear polarity. Myometrial invasion and “gaping gland” appearance is characteristic of SC.
Another important differential diagnosis is low grade EEC with papillary (villoglandular) pattern which shows elongated villous papillae. Complex papillary pattern, papillary tufting, pseudostratification, pleomorphism, and macronucleoli will be absent unlike SC. SC may also be confused with low grade EEC with small nonvillous papillae which contains small papillae within the endometrioid glands. The papillae are composed of buds of cells unlike true papillae of SC with large eosinophilic cytoplasm, and low grade features [6].
It is important to correctly diagnose uterine SCs as they are belligerent tumours. Initial presentation of the patient is usually at a higher stage. Surgery is the treatment of choice. Chemotherapy is less effective in uterine SC compared to that in SC in fallopian tube or ovary. For all the stages survival rate is 30-40%. SC present only in the the endometrium or selectively in an endometrial polyp has a better prognosis and upto 80% survival rate. Tumour size and presence of lymphovascular invasion are also important prognostic markers. The tumour being aggressive may infiltrate cervix, adnexa, lymph nodes, pleura, and peritoneum [6].
Conclusion
The diagnosis of serous papillary EC should not be missed as it is a very aggressive tumour. They can present with atypical associations like a small, infiltrative growth in lower uterine segment, not involving endocervical mucosa and with unusual associations of senile cystic endometrial atrophy and chronic myometritis. Endometrial biopsy should be undertaken for all women with atypical uterine bleeding even if the endometrial thickness is normal radiologically. If the preoperative evaluation by endometrial curating or biopsy detects a serous carcinoma in the endometrial lesions, full surgical exploration should be done to identify any metastasis.
[1]. Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trialThe Lancet 2000 355(9213):1404-11.10.1016/S0140-6736(00)02139-5 [Google Scholar] [CrossRef]
[2]. Kawata M, Miyoshi A, Fujikawa E, Kanao S, Takeda M, Mimura M, Serous endometrial intraepithelial carcinoma: case report and literature reviewJ Clin Gynecol Obstet 2017 6(2):49-52.10.14740/jcgo446w [Google Scholar] [CrossRef]
[3]. Clement PB, Young RH, Non-endometrioid carcinomas of the uterine corpus: a review of their pathology with emphasis on recent advances and problematic aspectsAdv Anat Pathol 2004 11(3):117-42.10.1097/00125480-200405000-00001 [Google Scholar] [CrossRef]
[4]. Matthews RP, Hutchinson-Colas J, Maiman M, Fruchter RG, Gates EJ, Gibbon D, Papillary serous and clear cell type lead to poor prognosis of endometrial carcinoma in black womenGynecol Oncol 1997 65(2):206-12.10.1006/gyno.1997.46179159326 [Google Scholar] [CrossRef] [PubMed]
[5]. Kurman RJ, Carcangiu ML, Herrington CS, WHO classification of tumours of the female reproductive organs 2014 Lyon, FranceIARC press [Google Scholar]
[6]. Gatius S, Matias-Guiu X, Practical issues in the diagnosis of serous carcinoma of the endometriumMod Pathol 2016 29(S1):S45-58.10.1038/modpathol.2015.14126715173 [Google Scholar] [CrossRef] [PubMed]
[7]. Faratian D, Stillie A, Busby-Earle R, Cowie VJ, Monaghan H, A review of the pathology and management of uterine papillary serous carcinoma and correlation with outcomeInt J Gynecol Cancer 2006 16(3):972-78.10.1136/ijgc-00009577-200605000-0000316803471 [Google Scholar] [CrossRef] [PubMed]
[8]. Lim D, Oliva E, Nonendometrioid endometrial carcinomasSemin Diagn Pathol 2010 27(4):241-60.10.1053/j.semdp.2010.09.00421309259 [Google Scholar] [CrossRef] [PubMed]
[9]. Darvishian S, Hummer AJ, Thaler HT, Bhargava R, Linkov I, Asher M, Serous endometrial cancers that mimic endometrioid adenocarcinomas: a clinicopathologic and immunohistochemical study of a group of problematic casesAm J Surg Pathol 2004 28(12):1568-78.10.1097/00000478-200412000-0000415577675 [Google Scholar] [CrossRef] [PubMed]
[10]. Bartosch C, Lopes JM, Oliva E, Endometrial carcinomas: a review emphasizing overlapping and distinctive morphological and immunohistochemical featuresAdv Anat Pathol 2011 18(6):415-37.10.1097/PAP.0b013e318234ab1821993268 [Google Scholar] [CrossRef] [PubMed]
[11]. Garg K, Soslow RA, Strategies for distinguishing low-grade endometrioid and serous carcinomas of endometriumAdvAnat Pathol 2012 19(1):1-10.10.1097/PAP.0b013e318234ab3622156830 [Google Scholar] [CrossRef] [PubMed]
[12]. Soslow RA, High-grade endometrial carcinomas-strategies for typingHistopathology 2013 62(1):89-110.10.1111/his.1202923240672 [Google Scholar] [CrossRef] [PubMed]
[13]. Gilks CB, Oliva E, Soslow RA, Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinomaAm J Surg Pathol 2013 37(6):874-81.10.1097/PAS.0b013e31827f576a23629444 [Google Scholar] [CrossRef] [PubMed]