Primary intraventricular haemorrhage is defined as bleeding in the ventricular system without a discernable parenchymal component or arising within 15 mm from the ventricular wall [1,2], arising from an intraventricular source or a lesion contiguous to the ventricles. Approximately, 70% of IVHs are secondary; secondary IVHs may occur as an extension of an intraparenchymal haemorrhage or SAH into the ventricular system. PIVH was defined for the first time by Sanders E in 1881 as “the flooding of the ventricle by blood without the presence of any rupture or laceration in the ventricular wall’’ [3]. The incidence of PIVH is 3.1% among all the patients with intracranial haemorrhage. This rises to 9% among the patients with intraparenchymal haematoma. Ventricular blood clots blocking Cerebrospinal Fluid (CSF) conduits cause acute obstructive hydrocephalus. Blood degradation products become embedded in the arachnoid granulations and may cause permanent occlusion and scarring, inhibiting CSF absorption and causing non obstructive (communicating) hydrocephalus. Spontaneous resorption and rebleeding may be seen. Diabetes mellitus, coagulopathy as an underlying aetiologic factor and presence of blood in all ventricles predict early mortality [4]. Hypertension along with AVMs, aneurysms, MMD, coagulopathy, and arteriovenous fistula are common associated risk factors [5]. Sudden onset of headache, nausea, vomiting together with alteration of the mental state and/or level of consciousness is the cardinal features of IVH. The pupillary and extraocular movement abnormalities, focal motor deficits, hyperreflexia, seizures, and stiff neck were less frequently seen. Focal neurological deficits are either minimal or absent in PIVH. There is a paucity of studies evaluating predictors of outcome in PIVH. With this study we tried to evaluate aetiological profile and predictors of outcome of PIVH.
The aim of this study is to evaluate clinical features of PIVH, predisposing risk factor, aetiology, radiological features and yield of diagnostic cerebral angiography in identifying the aetiological causes and also explicating the factors affecting the prognosis of the patients from Eastern India.
Materials and Methods
This cross-sectional study was started on March 2015 and continued up to October 2016 and the study protocol was approved by Institutional Ethical Committee of SSKM, IPGMER. The study population comprised of patients admitted in the out patient department and emergency departments of Bangur Institute of Neuroscience, IPGMER. PIVH was diagnosed on the basis of CT scan. After receiving an informed written consent for participation from the patient, they were included in the study. Those with intraparenchymal haemorrhage, SAH, IVH associated with trauma were excluded.
A total of 40 patients of PIVH of both male and female were recruited for the study. Data were collected by oral questionnaire method regarding relevant history from patient or his guardian/relative about signs and symptoms of stroke include vertigo, vomiting, facial deviation, weakness of particular half of body, convulsion. Pulse, BP, respiratory rate and temperature was measured. Pupil examination was done along with ophthalmoscopy to rule out papilloedema. Neurological examination done with proper evaluation of higher mental function, cranial nerve, tone, power, jerks and plantar. Cardiovascular examination done with proper emphasis on thrill, murmur and heart sounds. Poor outcome was defined as death, directly related to the PIVH or survival with significant neurologic deficits requiring institutional care. Good outcome was defined as survival with no or minimal neurologic deficits and functional independence [1]. We recorded the following clinical data (variables): i) individual characteristics and risk factors-sex, age, smoking, history of arterial hypertension (systolic pressure higher than 150 mmHg or diastolic higher than 90 mmHg or the patient was under antihypertensive therapy), diabetes (previous diagnosis of diabetes and/or past or present use of antidiabetic agents or need of antidiabetic treatment on discharge), previous stroke (ischaemic or haemorrhagic) and use of antiplatelet or anticoagulant medication; ii) clinical features at onset; iii) initial neurological examination and clinical parameters on admission such as blood pressure, Glasgow Coma Scale (GCS); iv) initial features of PIVH (ventricles involved, presence of hydrocephalus); and v) clinical course and outcome.
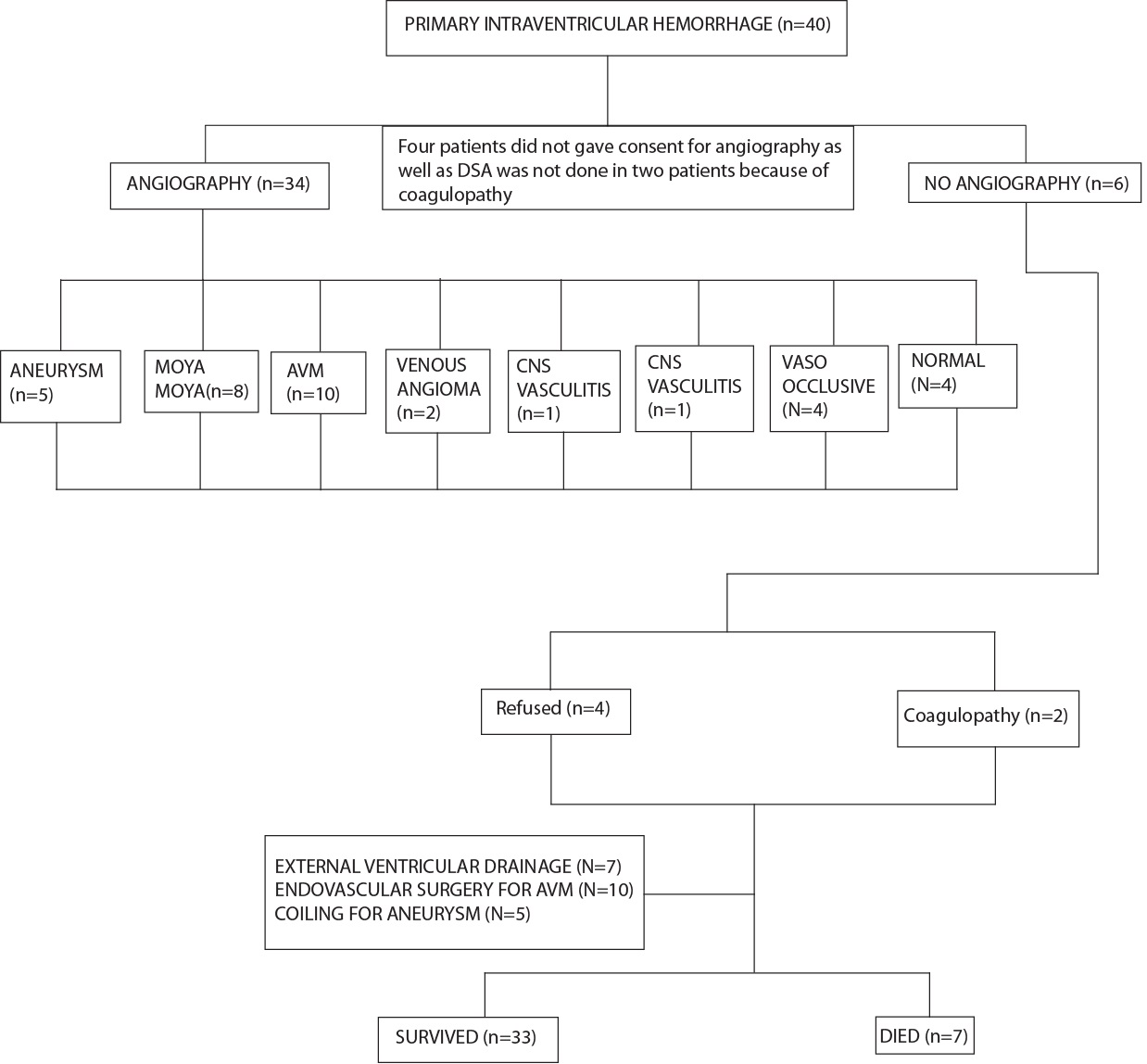
Angiography was done where feasible with special preference given to DSA. All patients were interviewed by telephone to identify neurological sequelae and educational or occupational achievement after the haemorrhage. Patients who survived were followed up by clinic visits. All records regarding subsequent hospitalisation and death were reviewed and relevant events occurring during follow-up were recorded. Study design is summarised in [Table/Fig-1].
Statistical Analysis
For statistical analysis, data were entered into a Microsoft excel spreadsheet and then analysed by SPSS 20.0.1 and GraphPad Prism version 5. Data have been summarised as mean and standard deviation for numerical variables and count and percentages for categorical variables. The median and the interquartile range have been stated for numerical variables that are not normally distributed. Student’s independent sample’s t-test was applied to compare normally distributed numerical variables between groups; unpaired proportions were compared by chi-square test or Fisher’s-exact test, as appropriate. A p-value ≤0.05 was considered as statistically significant.
Results
Majority of the study group consisted of male subjects who were non smoker, hypertensive, non diabetic [Table/Fig-2], in fourth decade of their life followed by third and sixth decade. According to GCS the patients were divided in three groups-GCS 3-4, 5-12, 13-15 and majority of the patients presented with GCS 13-15 [6]. With the improvement of GCS score survival rate gradually increased and mortality rate gradually decreased [Table/Fig-3]. Majority of the patients survived in present study population with PIVH. Clinical features presented by them are summarised in [Table/Fig-4]. Onset was sudden in 30 patients and fluctuating or progressive in 10. CT was performed on all patients and initial CT revealed extravasation of blood into all four ventricles in seven patients (17.5%), both lateral and third ventricles in 11 patients, both lateral ventricles in 16 patients and to the third and fourth ventricles in four patients and only in third ventricle in two patients. DSA was performed in 34 patients, with normal results in four and AVM detected in 10 patients (25%), MMD in eight patients (20%), aneurysm in five patients (12.5%), vaso occlusive disease in four patients (10%), venous angioma in two patients (5%), CNS vasculitis in one patient (2.5%). No cause was detected in 10 patients. Four patients did not give consent, DSA was not done in two patients because of coagulopathy and other four were having normal DSA. Other probable causative factors were hypertension in 24 patients. Location of AVM were paramedian (n=1), posterior paraventricular (n=1), frontal (n=1), parieto-occipital (n=2), temporo-occipital (n=1), intraventricular (n=2), high parietal (n=2) in present study. In present study, Posterior Inferior Cerebellar Artery (PICA) and Posterior Communicating Artery (PCOM) aneurysm was detected in one patient each, Anterior Communicating Artery (ACOM) aneurysm in two patients and rest had aneurysm in postero lateral aspect of communicating aspect of left ICA. Vaso-occlusive disease profile was as follows-Middle Cerebral Artery occlusion in one patient, ACA Occlusion in one patient, steno occlusive disease in distal most part of C7 segment of right ICA with collateralisation in right anterior circulation in one patient, total occlusion of right ICA distal to right ophthalmic artery with corticocortical collateral between right PCA and MCA in one patient.
| Salient feature | Number and percentage |
|---|
| Sex | Male-24 (60%), Yes | Female-16 (40%) No |
| Hypertension | 24 (60%) | 16 (40%) |
| Diabetes | 4 (10%) | 36 (90%) |
| Smoker | 14 (35%) | 26 (65%) |
| Hydrocephalus | 7 (17.5%) | 33 (82.5%) |
| Outcome | Dead-7 (17.5%) | Survived-33 (82.5%) |
| GCS | No. of patients (n=40) | Survival (%) [group specific] | Death (%) [group specific] |
|---|
| 3-4 (1st group) | 1 | 0 | 1 (100%) |
| 5-12 (2nd group) | 9 | 3 (33.33%) | 6 (66.67%) |
| 13-15 (3rd group) | 30 | 30 (100.0%) | 0 |
Presenting clinical features of IVH.
| Clinical feature | Number of cases (%) |
|---|
| Headache | 12 (30%) |
| Nausea/vomiting | 11 (27.5%) |
| Loss of consciousness | 7 (17.5%) |
| Confusion | 9 (22.5%) |
| Seizure | 4 (10%) |
| Vertigo | 7 (17.5%) |
| Hemiparesis | 8 (20%) |
| Facial paresis | 4 (10%) |
| Pupillary abnormality | 3 (7.5%) |
| Eom abnormality | 8 (20%) |
| Ataxia | 4 (10%) |
| Nuchal rigidity | 4 (10%) |
PIVH patients had associated early hydrocephalus (n=7, 17.5%) and these seven patients required external ventricular drainage. Early hydrocephalus was associated with poor prognosis as seven patients who developed hydrocephalus in this study all died. Medical complications included gastrointestinal bleeding, pneumonia, supraventricular tachycardia, acute renal insufficiency, hypokalemia, hypernatremia and hyponatremia, each of which was observed.
All patients were managed in an ICU or a recovery unit facility, with aggressive haemodynamic support, measures to decrease intracranial pressure and, when indicated, mechanical ventilation. For outcome assessment, the variables that appeared as significant univariate predictors of inhospital mortality were age, smoking, GCS score, distribution of ventricular bleed, hypertension, and hydrocephalus are statistically significant predictors of outcome [Table/Fig-5].
Univariate analysis of predictors of outcome with various parameters.
| Parameters | p-value |
|---|
| Age | 0.0013* |
| Sex | 0.1262 |
| Hypertension | 0.0173* |
| Diabetes | 0.6773 |
| Smoking | 0.0261* |
| Distribution of bleed | <0.0001* |
| Hydrocephalus | <0.0001* |
| Gcs | <0.0001* |
| Aetiology | 0.3443 |
statistically significant (p-value ≤0.05 was considered for statistically significant)
Discussion
In present study age, smoking, GCS score, distribution of ventricular bleed, hypertension and hydrocephalus are statistically significant predictors of outcome.
Patients were aged between 15 to 62 years (mean age=40.82), no patient in first decade or in eighth or ninth decade were included. Mean age in other studies were 60.6 years [2], 53 [5], 60 [7], 57.6 [8], 78.9 [9], 56 [10] respectively. Male predominance was reported in previous series [2,10] whereas, in some series female predominance was noticed [1,11]. Also, there was a male preponderance in this present study. Hypertension is a common predisposing factor for PIVH, with varied association as found in different studies, 38.4-80% [11,12]. In this present study, hypertension was the main predisposing factor and accounted for 60% (n=40) of patients.
Some investigators have used the extent of haematoma to determine the prognosis. Angelopoulos M et al., reported relatively good outcome as eight patients had haematoma in all ventricles and only 2 (25%) of these had poor outcomes [1]. Study done by Hameed B et al., showed the presence of blood in all ventricles which were found to be predictors of death [10]. In this present study, there was significant correlation between the extent of blood and the outcome. Among seven patients who had haematoma in all ventricles, six died and only one survived.
Giray S et al., in their study obtained majority of the patients have of all ventricular bleed (41%) followed by bleed in lateral ventricle (33%) [2]. Passero S et al., in their study obtained similar incidence of bleed in lateral ventricle (combining both and single lateral ventricle) and all ventricular bleed (42%) [8]. However, in present study majority of patients had bleeding in lateral ventricle followed by lateral and third ventricular bleed followed by all ventricular bleed [Table/Fig-6]. This difference might be due to difference in aetiology of PIVH.
Distribution of patients according to ventricular bleed.
| Distribution | Number | Percent |
|---|
| 3rd ventricle | 2 | 5.0% |
| 3rd, 4th ventricle | 4 | 10.0% |
| All ventricles | 7 | 17.5% |
| Lateral ventricle | 16 | 40.0% |
| Lateral, 3rd ventricle | 11 | 27.5% |
| Total | 40 | 100.0% |
In a study done by Hameed B et al., 33% of patients had diabetes mellitus and, it was found to be a significant predictor of death [10]. However, the presence of diabetes is not statistically significant predictor as an outcome in present study.
Similar to present study, other studies indicate headache as the most common presenting feature, followed by loss of consciousness [11,13]. Only 10% had generalised tonic clonic seizure. In the study by Passero S et al., seizure incidence was about 11.5% [8]. Another study done by Marti-Fabregas J et al., it was 23% [7]. In present study, incidence of seizure was 10%. Second common feature was nausea and vomiting following headache in this study. Angelopoulos M et al., and Srivastava T et al., reported similar observation in their study. 20% patients in this study presented with extraocular movement abnormality [1,5], whereas another study done by Angelopoulos M et al., it was 50% [1].
Thirty patients experienced acute onset, however in 10 patients onset was subacute, developing over hours or days. This subacute onset has been described previously [14]; however, other series have reported acute onset in all patients [1]. Although, it may be suggested by the clinical data, the clinical diagnosis of PIVH is often difficult and is rarely suspected before CT. High index of suspicion is necessary to clinch the diagnosis.
With improvement of GCS score, survival increases and mortality decreases. Similar to present study, Passero S et al., [8] in their study got GCS as significant univariate predictor of inhospital mortality. The prognosis of PIVH has altered as our diagnostic ability has improved, enabling to detect more benign cases. Poor prognosis and death are thought to occur inevitably from fatal brainstem dysfunction. Inhospital mortality in this present study was 17.5%, that reported in other series were 36% [1] and 42% [Table/Fig-2] [2,8].
After hypertension, AVM is an important cause of PIVH and varied between 14.2 and 32% [5,13] and in our series it accounted for (n =10) 25% of the aetiology. PIVH can be the presenting feature of MMD [15,16]. In present study, eight patients had MMD as aetiology of PIVH. Even after extensive investigations, no cause could be established in 16.7-26.92% of patients [7,15]. In our series, cause was not found in four patients (10%) even after cerebral angiography. Study done by Giray S et al., revealed that most PIVH patients had associated early hydrocephalus (58%) [2]. Passero S et al., showed 50% patient developed hydrocephalus in their study [8]. Study done by Bhattathiri PS et al., revealed 55% patients developed hydrocephalus as well as study done by Hameed B et al., showed 73% patients developed hydrocephalus [4,10]. Hydrocephalus occurred in 33.3% patients in the study done by Waleed Fawzy El-Saadany WF et al., however in present study only 17.5% patients developed hydrocephalus [17]. This low incidence of hydrocephalus in our study may be due to low amount of blood or may be due to ethnic or racial variation [18]. Few studies have been done so far to evaluate prognostic factors of primary IVH [8,19]. Passero S et al., showed GCS ≤8 and early hydrocephalus as independent predictors of inhospital mortality [8]. In the study done by Giray S et al., age, sex and hypertension did not have any impact on the outcome but GCS and development of early hydrocephalus were estimated to be prognostic risk factors [2]. Hameed B et al., observed that diabetes mellitus, coagulopathy, blood in all ventricles were predictors of death during initial hospital stay and hydrocephalus was predictor for poor outcome [10]. El-Saadany WF et al., observed that, admission GCS severity of IVH, number of ventricles filled with blood, and presence of acute hydrocephalus correlated with prognosis of PIVH [17]. Level of consciousness on admission was an important prognostic factor in the study done by Marti-Fabregas J et al., [7]. In present study age, smoking, GCS score, distribution of ventricular bleed, hypertension, hydrocephalus are statistically significant predictors of outcome on univariate analysis [Table/Fig-5,6].
Limitation
Study was limited by its small sample size. Study subjects may not be representative of the general elderly population of India, as it was a hospital-based study. A population-based survey will be more informative. Again, multivariate logistic regression analysis was not possible in this study due to small proportion of population that dies or may be due to small sample size. It could be possible if a larger sample size over a longer duration is studied.
Conclusion
Hypertension is the most commonly associated risk factor for PIVH followed by vascular malformations. Cerebral angiography is highly useful in aetiological diagnosis of PIVH. Age, GCS, distribution of ventricular bleed, smoking, hypertension and hydrocephalus are correlated with outcome. IVH may be a devastating complication associated with ICH, predicting worsened morbidity and mortality. Awareness of possible causes is important in order to guide patient management. Upon identification of IVH, CT, MRI/MR angiography and catheter angiography can provide complementary information for a confident evaluation and a potential treatment access. The present study, apart from clinical feature, aetiology, angiographic finding in PIVH also provides a valuable insight into the stroke disorder of this country.
statistically significant (p-value ≤0.05 was considered for statistically significant)