Introduction
Polycystic Ovarian Disease (PCOD) is the most common hormonal disorder in women. PCOD is associated with an increase in subclinical atherosclerotic disease and endothelial dysfunction. The altered endothelial function and early endothelial damage can be assessed by Urinary Albumin Excretion (UAE), a marker of an atherogenic background. Dyslipidaemia is a very common metabolic abnormality in women with PCOD due to elevated androgen level and insulin resistance. As evidenced from previous studies PCOD patients are at an increased cardiovascular risk compared with the age matched controls.
Aim
To evaluate the prevalence of microalbuminuria and dyslipidaemia in premenopausal PCOD patients compared to normal premenopausal women.
Materials and Methods
The present study was carried out at Department of Biochemistry, Aarupadai Veedu Medical College and Hospital Puducherry, India. A total of 40 diagnosed PCOD patients (according to Rotterdam criteria) of premenopausal age (21 to 42 years) and 40 age and sex matched controls (22 to 43 years) without PCOD were included in the study. All subjects had undergone measurement of height, weight and Blood Pressure (BP), and detailed systemic examination. Fasting plasma glucose, serum cholesterol, Triglycerides (TG), and High Density Lipoprotein-Cholesterol (HDLc) were estimated by using commercially available kits in automated Chemistry Analyser (ChemWell). Low Density Lipoprotein-Cholesterol (LDLc) and Very Low Density Lipoprotein-Cholesterol (VLDLc) were calculated by Friedwald’s equation. Urine microalbumin was estimated by Latex turbidimetry method using semi Autoanalyser BIOTRON BTR830. Urine creatinine was estimated by commercial kit in Autoanalyser. Albumin-Creatinine Ratio (ACR) value more than 30 mg/g was taken as microalbuminuria positive. Student’s t-test and SPSS version 16.0 were used for statistical analysis.
Results
Out of 40 PCOD patients 21 patients were having microalbuminuria and out of 40 controls only two were having microalbuminuria. Routine biochemical investigations registered a significant rise of fasting plasma glucose, TG, TC, LDLc and VLDLc in PCOD patients, incomparison with controls (p=0.02 for LDLc and p<0.001 for all otherparameters). Significant alterations in lipid parameters showed association of dyslipidaemia in PCOD patients.
Conclusion
In the present study prevalence of microalbuminuria and dyslipidaemia are more in patients with polycystic ovary disease than age matched controls so these parameters can be frequently estimated to prevent complications in PCOD patients.
Introduction
Polycystic ovarian disease is the most common hormonal disorder in women worldwide, with an estimated prevalence between 4 and 8%, and one of the most common cause of ovulatory infertility [1]. PCOD is a heterogeneous disorder with unknown aetiology in which ovaries produces excess androgens and is associated with insulin resistance [2]. PCOD was diagnosed if any two of the following three criteria (Rotterdam criteria) were present after excluding other possible causes: i) oligo and/or anovulation; ii) clinical and/or biochemical signs of hyperandrogenism; and iii) polycystic ovaries on ultrasonography [3].
Insulin resistance is an important aspect of PCOD and may contribute to an increased risk of developing Type 2 diabetes and coronary heart disease [4].
The PCOD patients have endothelial dysfunction which leads to increase incidence of subclinical atherosclerotic disease [5,6]. Altered insulin regulation of endothelial Nitric Oxide (NO) synthesis leads to decreased NO production and impaired NO dependant vasodilatation which may be a cause for endothelial dysfunction [6,7]. The altered endothelial function and early endothelial damage can be assessed by UAE, a marker of an atherogenic background [8]. Epidemiologic and clinical evidence shows that microalbuminuria is associated with an increased cardiovascular mortality [9,10]. Many studies have shown that people having very low levels of microalbuminuria only without chronic diseases like renal dysfunction, hypertension, or diabetes suffer from coronary artery disease and death [11,12]. Dyslipidaemia is a very common metabolic abnormality in women with PCOD, with a prevalence of up to 70% [13]. Women with PCOD are usually obese and have elevated androgen level leading to dyslipidaemia [14].
Because of insulin resistance, they are expected to be at increased risk for dyslipidaemia [13]. Due to presence of cardiovascular risk factors like dyslipidaemia, insulin resistance, and endothelial dysfunction, PCOD patients are at an increased cardiovascular risk compared with the age matched controls [15]. Some studies had shown that women with PCOD have increased risk for cardiovascular events not related to Body Mass Index (BMI) [16,17]. Very few Indian studies are there and due to the increasing prevalence of PCOD, we decided to explore the association of microalbuminuria and dyslipidaemia in PCOD patients and to compare that with age matched controls [12,14].
Materials and Methods
The present comparative cross sectional hospital based study was carried out at Biochemistry Department, Aarupadaiveedu Medical College and Hospital, Puducherry, India from April 2016 to July 2016. The patients attended Obstetric and Gynaecology Out Patient Department and diagnosed as PCOD patient were selected for the study. A total of 40 diagnosed PCOD patients (according to Rotterdam criteria) of premenopausal age (21 to 40 years) and 40 age matched controls were taken for the study [3].
Controls were selected among the female patients attending the OPD of Medicine and Obstetric and Gynaecology department (who came for some common acute illness like viral fever, diarrhoea, dysmenorrhea) and also the faculties of AV Medical College and Hospital. The study group (both cases and control) was selected after excluding the exclusion criteria.
Sample size was calculated with the help of software based on other similar Study [18]. Both Scientific committee and Ethical committee clearance was taken and informed consent was taken from the study population.
The PCOD was diagnosed according to the Rotterdam criteria [3]. The patients having two or more of the following criteria were defined as PCOD:
History of oligo and/or anovulation in reproductive age.
Clinical and/or biochemical signs of hyperandrogenism: hirsutism score of >6 and/or high total testosterone level.
Typical ovarian imaging of polycystic ovaries on ultrasound: multiple follicles in each ovary measuring 2-9 mm in diameter and/or increased ovarian volume (>10 mL).
Inclusion Criteria
All newly diagnosed cases of PCOD from Department of Obstetrics and Gynaecology in the age group 21 to 40 years were taken as cases. Apparently healthy premenopausal age (21 to 40 years) women attending medicine and obstetrics and gynaecology OPD and staff of AVMC were taken as control. Controls were having regular menstrual cycles and with no clinical features of hyperandrogenism, thereby excluding the diagnosis of PCOD in this group.
Exclusion Criteria
Women with diabetes mellitus, hyperprolactinaemia, thyroid, renal, hepatic, adrenal and cardiac dysfunction were excluded from the study. Pregnancy, urinary tract infection, and those on lipid-lowering drugs, anti-hypertensive agents were also excluded from the study. Already known microalbuminuria and dyslipidemia cases were excluded from study group.
Height and weight were measured and BMI was calculated by the formula: weight in kg/height in m2 [19]. Blood pressure of the study subjects was measured by standardised methods. Both cases and controls were subjected to estimationof biochemical parameters. A 5 mL of venous blood was collected from anterior cubital vein of left hand in fluoride tube for glucose and clot activator tubes for other estimations after an overnight fast. Fasting plasma glucose, serum cholesterol, TG, and HDLc were estimated by using commercially available kits in automated Chemistry analyser (Chemwell). Serum LDLc and VLDLc were calculated by Friedewald’s equation {LDLc=Total Cholesterol–(HDLc+VLDLc), Where VLDLc=TG/5} [20].
Morning fasting urine samples were collected for micro albumin and creatinine estimation. Urine micro albumin was analysed in semi autoanalyser-BIOTRON BTR 830 and creatinine was estimated by auto analyser. ACR value more than 30 mg/g was taken as microalbuminuria positive.
Statistical Analysis
All the results were subjected to statistical analysis by the Statistical Package for the Social Sciences (SPSS) software 16.0 version. Student t-test was used for comparison among variables in two groups. A p-value less than 0.05 were taken as significant.
Results
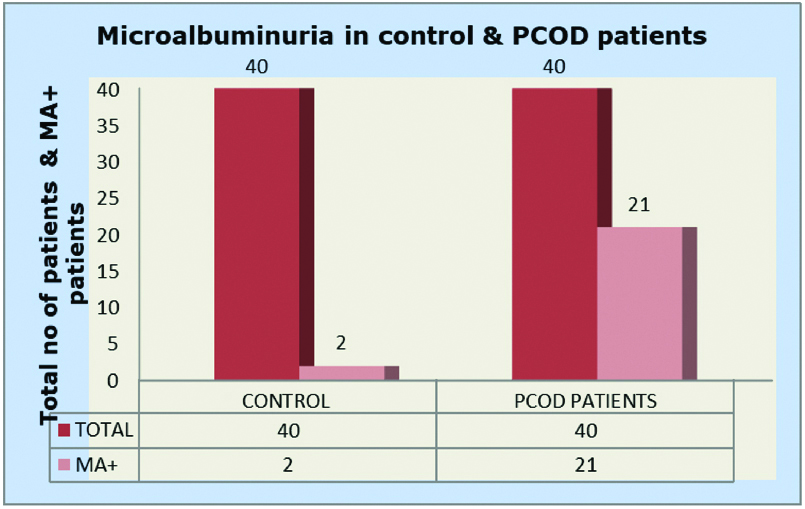
The age distribution of patients and controls were between 21-40 years [Table/Fig-1]. BMI of PCOD patients were found to be more than the controls (p<0.001) [Table/Fig-1]. Routine biochemical investigations registered a significant rise of fasting plasma glucose, TG, total cholesterol, LDLc and VLDLc in PCOD patients, in comparison with controls (p=0.02 for LDL cholesterol and p<0.001 for all other parameters) [Table/Fig-2]. Out of 40 PCOD patients 21 patients were having micro albuminuria and out of 40 controls only two were having micro albuminuria [Table/Fig-3].
Comparison of BMI and serum biochemical parameters in control and PCOD patients.
| Age in years | BMI (Kg/m2) | SBP (mm Hg) | DBP (mm Hg) | FBS (mg/dL) | Urea (mg/dL) | Creatinine (mg/dL) |
|---|
| Control group n=40 |
| Mean±SD | 32.8±5.5 | 22.9±4.1 | 116±8.7 | 73±6.4 | 95.9±10.9 | 22.9±5.9 | 0.75±0.2 |
| Range | 22-43 | 17.1-32 | 90-130 | 60-80 | 59-110 | 14-45 | 0.4-1.6 |
| PCOD group n=40 |
| Mean±SD | 30.4±6.3 | 24.9±3.3 | 119±8.7 | 73±5.6 | 150.1±59.6 | 24.2±9.9 | 0.91±0.3 |
| Range | 21-42 | 18.4-35.7 | 100-130 | 60-80 | 53-324 | 12.0-69 | 0.4-2.6 |
| Comparison between two groups |
| Control vs. PCOD patient | p=0.07 | p=0.02 | p=0.12 | p=1 | p<0.001 | p=0.2 | p=0.01 |
| ns | * | ns | ns | *** | ns | * |
ns=not significant; *significant; ***highly significant
Data analysis was done by SPSS Software 16.0 version. Student’s t-test was used for comparison among variables in two groups. A p-value less than 0.05 was taken as significant.
BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; FBS: Fasting blood sugar; PCOD: Polycystic ovarian disease
Comparison of lipid profile and ACR in control and PCOD patients.
| TC (mg/dL) | TG (mg/dL) | HDL c (mg/dL) | VLDL c (mg/dL) | LDL c (mg/dL) | UACR (mg/g) |
|---|
| Control group n=40 |
| Mean±SD | 183.6±24.6 | 103.9±42.6 | 37.6±6.62 | 20.77±8.48 | 125.3±25.8 | 13.82±8.9 |
| Range | 122-240 | 54-261 | 23-48 | 11.0-52 | 76-186 | 3.0-41 |
| PCOD group n=40 |
| Mean±SD | 216.5±38 | 170.9±84 | 40.92±10.4 | 34.1±16.7 | 141.4±37.2 | 47.17±39.8 |
| Range | 162-304 | 82-456 | 23-60 | 16-91 | 69-208 | 5-150 |
| Comparision between two groups |
| Control vs. PCOD patients | p<0.001 | p<0.001 | p=0.09 | p<0.001 | p=0.02 | p<0.001 |
| *** | *** | Ns | *** | * | *** |
ns=not significant; *significant; ***highly significant
Data analysis was done by SPSS Software 16.0 version. Student’s t-test was used for comparison among variables in two groups. A p-value less than 0.05 were taken as significant.
TC: Total cholesterol; TG: Triglyceride; HDLc: High density lipoprotein cholesterol; LDLc: Low density lipoprotein cholesterol; VLDLc: Very low density lipoprotein cholesterol; UACR: Urine albumin creatinine ratio; PCOD: Polycystic ovarian disease
Comparing microalbuminuria in control and PCOD patients.
MA+ Microalbuminuria Positive
Out of 40 controls only 2 were MA+
Out of 40 PCOD Patients 21 were MA+
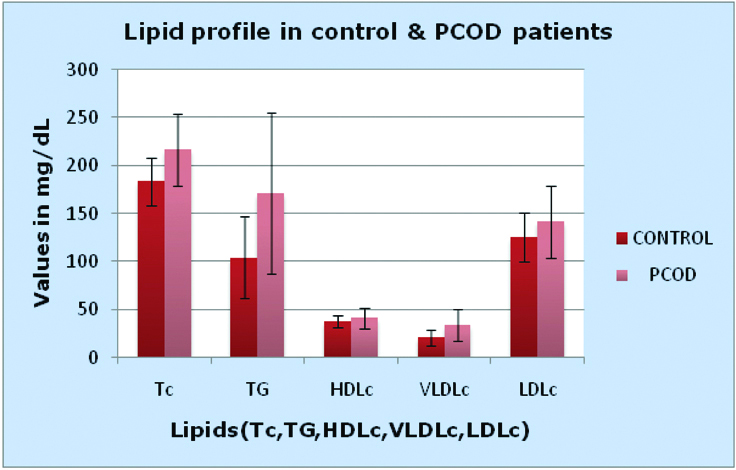
Significant alterations in lipid parameters showed association of dyslipidaemia in PCOD patients. There was a small rise in serum HDLc in PCOD patients (Mean±SD is 37.6±6.62 for controls compared to cases which is 40.92±10.4 and the p-value is 0.09) than in controls but it was not significant [Table/Fig-4]. ACR was more in PCOD patients than controls (p<0.001, which was highly significant) [Table/Fig-2].
Comparing lipid profile in control and PCOD patients.
TC: Total cholesterol (Mean±SD 183.6±24.6 for controls and 216.5±38 for PCOD patients); TG: Triglyceride (Mean±SD 103.9±42.6 for controls and 170.9±84 for PCOD patients); HDLc: High density lipoprotein cholesterol (Mean±SD 37.6±6.62 for controls and 40.92±10.4 for PCOD patients); VLDLc: Very low density lipoprotein cholesterol (Mean±SD 20.77±8.48 for controls and 34.1±16.7 for PCOD patients); LDLc: Low density lipoprotein cholesterol (Mean±SD 125.3±25.8 for controls and 141.4±37.2 for PCOD patients)
Discussion
Microalbuminuria is excretion of albumin in urine in the range of 20-200 μg/minute. It is often expressed as ACR and the range is 30-300 mg of albumin/g of creatinine in urine [21].
The PCOD patients usually have Insulin resistance which appears to play a key role in the development of endothelial damage [22]. Microalbuminuria and Leakage of other plasma macromolecules enhances inflammatory reactions which may initiate the process of atherosclerosis and increases the risk of CVDs. Some studies showed that the predictive power of microalbuminuria levels for cardiovascular risk were not dependent on other cardiovascular risk factors like diabetes and or hypertension but also present in otherwise healthy individuals [23,24]. Lowering albuminuria could decrease CVDs in PCOD patients [25]. The Framingham Heart Study found that six year risk of CVD was three fold higher in non-hypertensive, non-diabetic subjects with urinary ACR above the gender specific median than in those with urinary ACR below the median [26]. Hyperinsulinaemia often associated with PCOD may cause glomerular hypertension and increase filtration leading to increased albumin ultrafiltration and excretion [27]. Some studies got a clear association between more severe insulin resistance and microalbuminuria [28].
In present study, we got ACR value of 47.17±39.8 mg/g for PCOD patients compared to 13.82±8.9 mg/g for normal controls which is highly significant (p<0.001). This is similar to the report of Ganie MA et al., and Duleba AJ et al., [4,29].
The PCOD patients are usually obese and have elevated androgen and insulin levels, all these predispose them to develop dyslipidaemia. Moreover, they are insulin resistant too. Insulin resistance and hyperinsulinaemia are also associated with dyslipidaemia which initiates atherosclerosis [30,31]. Increased insulin concentrations increases VLDL synthesis, leading to hypertriglyceridaemia [32]. Progressive elimination of lipid and apolipoproteins from the VLDL particle leads to an increased formation of intermediate-density lipoprotein and LDL, both of which are atherogenic. Apart from hypertension and dyslipidaemia insulin is an independent risk factor for development of atherosclerosis. Insulin increases cholesterol transport into arteriolar smooth muscle cells and increases endogenous lipid synthesis by these cells. Insulin also stimulates the proliferation of arteriolar smooth muscle cells, augments collagen synthesis in the vascular wall, increases the formation of lipid plaques, and stimulates the production of various growth factors [30].
Insulin resistance present in PCOD patients appears to be associated with lipid abnormalities and atherosclerotic CVD. As a consequence, insulin resistance contributes to, high TG and high LDL. Reverse transport of cholesterol to liver is impaired, leading to its reduced excretion [33]. Therefore, these patients have increased serum levels of total cholesterol.
The PCOD women have increased androgen which is associated with increased hepatic lipase activity [34,35]. Hepatic lipase converts more buoyant HDLc to smaller denser HDLc which can be taken up by liver thereby decreasing HDLc level and it also converts more buoyant LDLc to smaller denser LDLc both are risk factors for CVD [36]. Thus, dyslipidaemia may precede the association with insulin resistance and increased risk for CVD. So preventing dyslipidemia in PCOD patients CVD can be prevented.
In the present study TG, TC, LDLc and VLDLc in PCOS patients were significantly higher in comparison with controls (p=0.02 for LDLc cholesterol and p<0.001 for all other parameters). Significant alterations in lipid parameters show association of dyslipidaemia in PCOS patients. There was a small rise in serum HDLc in PCOS patients than controls but it is not significant. This study finding is similar to the study of Ambiger S, Kim JJ and Choi YM and Kaviprasanna S et al., [37-39].
Hence, in PCOD women microalbuminuria and serum lipid profile can be done early and at frequent intervals to prevent the progression and complications of the disease.
Limitation
Limitations of present study were small sample size. We could not measure the extended lipid profile which is more reliable. Sample (blood) taken from the subjects once only.
Conclusion
Prevalence of microalbuminuria and dyslipidaemia are more in patients with PCOD patients than in age matched controls. PCOD women should be evaluated for status of serum lipids and microalbuminuria at frequent intervals to prevent the complications and which aids in the management also.
ns=not significant; *significant; ***highly significantData analysis was done by SPSS Software 16.0 version. Student’s t-test was used for comparison among variables in two groups. A p-value less than 0.05 was taken as significant.BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; FBS: Fasting blood sugar; PCOD: Polycystic ovarian disease
ns=not significant; *significant; ***highly significantData analysis was done by SPSS Software 16.0 version. Student’s t-test was used for comparison among variables in two groups. A p-value less than 0.05 were taken as significant.TC: Total cholesterol; TG: Triglyceride; HDLc: High density lipoprotein cholesterol; LDLc: Low density lipoprotein cholesterol; VLDLc: Very low density lipoprotein cholesterol; UACR: Urine albumin creatinine ratio; PCOD: Polycystic ovarian disease
[1]. Ehrmann DA, Polycystic ovary syndromeN Engl J Med 2005 352:1223-36.Available from: https://www.uptodate.com/contents/clinical-manifestations-of-polycystic-ovary-syndrome-in-adults/abstract/110.1056/NEJMra04153615788499 [Google Scholar] [CrossRef] [PubMed]
[2]. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO, The prevalence and features of the polycystic ovary syndrome in an unselected populationJ Clin Endocrinol Metab 2004 89(6):2745-49.10.1210/jc.2003-03204615181052 [Google Scholar] [CrossRef] [PubMed]
[3]. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group: Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndromeFertil Steril 2004 81:19-25.10.1016/j.fertnstert.2003.10.004 [Google Scholar] [CrossRef]
[4]. Ganie MA, Farooqui KJ, Bhat MA, Mir MM, Shah ZA, Douhath S, Pattern of urinary albumin excretion in normotensive young and adolescent Indian women with polycystic ovary syndromeIndian Endocrinol Metab 2012 16(2):277-82.10.4103/2230-8210.9375222470868 [Google Scholar] [CrossRef] [PubMed]
[5]. Diamanti-Kandarakis E, Spina G, Kouli C, Migdalis I, Increased endothelin 1 levels in women with polycystic ovary syndrome and the beneficial effect of metformin therapyJ Clin Endocrinol Metab 2001 86:4666-73.10.1210/jcem.86.10.790411600523 [Google Scholar] [CrossRef] [PubMed]
[6]. Kelly CJ, Speirs A, Gould GW, Petrie JR, Lyall H, Connell JM, Altered vascular function in young women with polycystic ovary syndromeJ Clin Endocrinol Metab 2002 87(1)(1):742-46.10.1210/jcem.87.2.819911836314 [Google Scholar] [CrossRef] [PubMed]
[7]. Lakhani K, Constantinovici N, Purcell WM, Fernando R, Hardiman P, Internal carotid-artery response to 5% carbon dioxide in women with polycystic ovariesLancet 2000 356:1166-67.10.1016/S0140-6736(00)02764-1 [Google Scholar] [CrossRef]
[8]. Feldt-Rasmussen B, Microalbuminuria, endothelial dysfunction and cardiovascular riskDiabet Metabol (Paris) 2000 26:64-66. [Google Scholar]
[9]. Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individualsJAMA 2001 286:421-26.10.1001/jama.286.4.42111466120 [Google Scholar] [CrossRef] [PubMed]
[10]. De Zeeuw D, Parving HH, Henning RH, Microalbuminuria as an early marker for cardiovascular diseaseJ Am Soc Nephrol 2006 17:2100-05.Available from: https://www.ncbi.nlm.nih.gov/pubmed/16825327?dopt=Abstract10.1681/ASN.200605051716825327 [Google Scholar] [CrossRef] [PubMed]
[11]. Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, Jensen G, Clausen P, Scharling H, Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetesCirculation 2004 110:32-35.10.1161/01.CIR.0000133312.96477.4815210602 [Google Scholar] [CrossRef] [PubMed]
[12]. Sukhija R, Aronow WS, Kakar P, Garza L, Sachdeva R, Sinha A, Relation of microalbuminuria and coronary artery disease in patients with and without diabetes mellitusAm J Cardiol 2006 98:279-81.10.1016/j.amjcard.2006.01.09816860009 [Google Scholar] [CrossRef] [PubMed]
[13]. Legro RS, Kunselman AR, Dunaif A, Prevalence and predictors of dyslipidemia in women with polycystic ovary syndromeAm J Med 2001 111:607-13.10.1016/S0002-9343(01)00948-2 [Google Scholar] [CrossRef]
[14]. Latha MM, Bhaskar MV, Sharma SSB, Sumapreethi A, Marker of oxidative stress and serum lipids in patients with polycystic ovarian syndromeJ Evol Med Dent Sci 2012 1(5):769-74.10.14260/jemds/123 [Google Scholar] [CrossRef]
[15]. Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JM, Sattar N, Low grade chronic inflammation in women with polycystic ovarian syndromeJ Clin Endocrinol Metab 2001 86:2453-555.10.1210/jcem.86.6.758011397838 [Google Scholar] [CrossRef] [PubMed]
[16]. de Groot PCM, Dekkers OM, Romijn JA, Dieben SWM, Helmerhorst FM, PCOS, coronary heart disease, stroke and the influence of obesity: a systematic review and meta-analysisHuman Repro Update 2011 17(4):495-500.10.1093/humupd/dmr00121335359 [Google Scholar] [CrossRef] [PubMed]
[17]. Chiu WL, Boyle J, Vincent A, Teede H, Moran LJ, Cardiometabolic risks in polycystic ovary syndrome: non-traditional risk factors and the impact of obesityNeuroendocrinol 2017 104:412-24.10.1159/00045523328006770 [Google Scholar] [CrossRef] [PubMed]
[18]. Saha Indranil, Paul Bobby, Essentials of biostatistics 2016 2nd EditionAcademic Publishers:64-65. [Google Scholar]
[19]. Nevill A, Holder R, Body mass index: a measure of fatness or leanness?Brit J Nutri 1995 73(4):507-16.10.1079/BJN199500557794868 [Google Scholar] [CrossRef] [PubMed]
[20]. Friedewald WT, Levy R, Fredrickson DS, Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifugeClin Chem 1972 18:499-502. [Google Scholar]
[21]. Bakker AJ, Detection of microalbuminuria. Receiver operating characteristic curve analysis favors albumin-to-creatinine ratio over albumin concentrationDiabetes Care 1999 22(2):307-13.10.2337/diacare.22.2.30710333950 [Google Scholar] [CrossRef] [PubMed]
[22]. McFarlane SI, Banerji M, Sowers JR, Insulin resistance and cardiovascular diseaseJ Clin Endocrinol Metabol 2001 86(2):713-18.10.1210/jcem.86.2.720211158035 [Google Scholar] [CrossRef] [PubMed]
[23]. Romundstad S, Holmen J, Hallan H, Kvenild K, Krüger O, Midthjell K, Microalbuminuria, cardiovascular disease and risk factors in a nondiabetic/ nonhypertensive population. The Nord-Trondelag Health Study (HUNT, 1995- 97), NorwayJ Inter Med 2002 252(2):164-72.10.1046/j.1365-2796.2002.01025.x12190892 [Google Scholar] [CrossRef] [PubMed]
[24]. Stehouwer CDA, Smulders YM, Microalbuminuria and risk for cardiovascular disease analysis of potential mechanismsJ Am Soc Nephrol 2006 17:2106-11.10.1681/ASN.200512128816825333 [Google Scholar] [CrossRef] [PubMed]
[25]. Basi S, Fesler P, Mimran A, Lewis JB, Microalbuminuria in Type 2 Diabetes and Hypertension A marker, treatment target, or innocent bystander?Diabetes Care 2008 31(2):S194-201.10.2337/dc08-s24918227485 [Google Scholar] [CrossRef] [PubMed]
[26]. Yin J, Li M, Xu L, Wang Y, Cheng H, Zhao X, Insulin resistance determined by Homeostasis Model Assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagersDiabetol Metabol Syndr 2013 5(1):7110.1186/1758-5996-5-7124228769 [Google Scholar] [CrossRef] [PubMed]
[27]. Li XH, Lin HY, Wang SH, Guan LY, Wang YB, Association of Microalbuminuria with Metabolic Syndrome among aged populationBio Med Res Int 2016 :1-7.10.1155/2016/924127827200378 [Google Scholar] [CrossRef] [PubMed]
[28]. Arnlov J, Evans JC, Meigs JB, Wang TJ, Fox CS, Levy D, Low grade albuminuria and incidence of cardiovascular disease events in non-hypertensive and non-diabetic individuals the Framingham Heart StudyCirculation 2005 112:969-75.10.1161/CIRCULATIONAHA.105.53813216087792 [Google Scholar] [CrossRef] [PubMed]
[29]. Duleba AJ, Ahmed IM, Predictors of urinary albumin excretion in women with polycystic ovary syndromeFertil Steril 2010 93:2285-90.10.1016/j.fertnstert.2008.12.12019217093 [Google Scholar] [CrossRef] [PubMed]
[30]. Chapman MJ, Sposito AC, Hypertension and dyslipidaemia in obesity and insulin resistance: pathophysiology, impact on atherosclerotic disease and pharmacotherapyPharmacol Ther 2008 117(3):354-73.10.1016/j.pharmthera.2007.10.00418215759 [Google Scholar] [CrossRef] [PubMed]
[31]. Bornfeldt KE, Tabas I, Insulin resistance, hyperglycemia, and atherosclerosisCell Metabol 2011 14(5):575-85.10.1016/j.cmet.2011.07.01522055501 [Google Scholar] [CrossRef] [PubMed]
[32]. Verges B, Pathophysiology of diabetic dyslipidaemia: where are we?Diabetologia 2015 58(5):886-99.10.1007/s00125-015-3525-825725623 [Google Scholar] [CrossRef] [PubMed]
[33]. Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH, The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosisJ Lipid Res 2009 50(Supp):S189-94.10.1194/jlr.R800088-JLR20019064999 [Google Scholar] [CrossRef] [PubMed]
[34]. Valkenburg O, Steegers-Theuniseen RP, Smedts HP, Dallinga-Thie GM, Fauser BC, Westerveld EH, A more atherogenic serum lipoprotein profile is present in women with polycystic ovary syndrome: a case-control studyJ Clin Endocrinol Metab 2008 93(2):470-76.10.1210/jc.2007-175618056772 [Google Scholar] [CrossRef] [PubMed]
[35]. Tangvarasittichai S, Poonsub P, Tangvarasittichai O, Association of serum lipoprotein ratios with insulin resistance in type 2 diabetes mellitusIndian J Med Res 2010 131:641-48. [Google Scholar]
[36]. Herbst KL, Amory JK, Brunzell JD, Chansky HA, Bremner WJ, Testosterone administration to men increases hepatic lipase activity and decreases HDL and LDL size in 3 wkAm J Physiol Endocrinol Metab 2003 284(6):E1112-18.10.1152/ajpendo.00524.200212736156 [Google Scholar] [CrossRef] [PubMed]
[37]. Ambiger S, Study of insulin resistance and lipid profile in polycystic ovarian syndromeInt J Sci Res Pub 2016 6(2):1-5. [Google Scholar]
[38]. Kim JJ, Choi YM, Dyslipidemia in women with polycystic ovary syndromeObstet Gynecol Sci 2013 56(3):137-42.10.5468/ogs.2013.56.3.13724327994 [Google Scholar] [CrossRef] [PubMed]
[39]. Kaviprasanna S, Saikumar P, Alaguveni T, Insulin resistance as an independent risk factor for the development of dyslipidemia in polycystic ovarian syndromeAdv Biol Res 2014 8(2):53-56. [Google Scholar]