Introduction
During the past few decades, the unrestrained usage of antimicrobial agents for treating various infections, as growth promoters in animal industry, agricultural industry and in other food industries, have resulted in a worrisome development of emergence of antimicrobial resistance [1,2]. The MDR and Extreme Drug Resistant (XDR) pathogens have become a substantial therapeutic challenge to all the infection control practitioners in healthcare centres [3]. Persistent colonization of bacteria in urinary tract without symptoms is termed as Asymptomatic Bacteriuria (ASB), a subset of Urinary Tract Infection (UTI) and clinically applies to the presence of ≥100,000 CFU of a single organism [4].
The chances of acquiring bacteriuria vary during the course of pregnancy and it ranges from 0.8% in the early weeks to 1.93% towards the end of pregnancy [5]. UTI are more common during pregnancy due to the physiological and morphological changes happening in these patients [6]. Smooth muscle relaxation results in decreased peristalsis of the ureters, increased bladder capacity and urinary stasis [7]. In addition, change in urinary pH, aminoaciduria, pregnancy-induced glycosuria will promote bacterial growth [8]. Significant reduction in immune responses and also increase in plasma volume during pregnancy supports the growth of both commensal and pathogenic bacteria [7]. However, the outcomes of ASB are very critical which includes maternal and fetal morbidity and mortality [9]. Following appropriate treatment, the risk of pyelonephritis and low birth weight infant declines in 25-35% of asymptomatic bacteriuria cases [10].
Ampicillin, Amoxicillin, Nitrofurantoin, Trimethoprim-sulfamethoxazole, Cefuroxime, Cephalexin, and other oral cephalosporins were the drugs of choice for treating ASB, but production of ESBL through acquisition of ESBL encoding genes by these Gram Negative Bacilli (GNB), have made these antimicrobials ineffective [11]. MDR Gram-negative bacilli of the Enterobacteriaceae family have been increasingly responsible for infections among neonates causing both early and late onset sepsis [12]. Hence, it becomes indispensable to conduct a proper antenatal screening to prevent the untoward effects of UTI. In recent times as there is an increase in the resistant pathogens among the community, scrutiny of their antibiogram with molecular analysis would enable the clinicians to tackle these issues in the near future. Thus, this study was aimed to investigate the incidence of ASB among the antenatal mothers and the prevalence rate of ESBL production among the gram-negative isolates, which is followed by their molecular characterization.
Materials and Methods
A prospective cohort study was conducted at Mahatma Gandhi Medical College and Research Institute (MGMCRI), a tertiary care teaching hospital in Puducherry, India, over a period of three months from June 2015 to August 2015. All antenatal women visiting the Antenatal Clinic (ANC) during the study period, for their routine checkup for safe confinement with no signs of UTI e.g., no fever, chills with rigor, burning micturition, loin pain, dysuria etc., were included in this study. During the routine periodic out-patient ANC checkup, their mid-stream clean catch urine was collected in a sterile container (morning sample) and was processed. Following wet mount urine examination, urine samples with significant number of WBCs and bacteria were then got plated and further studied. Pregnant women who were on antibiotic treatment two weeks prior to their initial visit were excluded from the investigation [13]. Those pregnant women with clinical signs and symptoms of UTI, pregnancy induced diabetes mellitus, hypertension, congenital anomalies of the urinary tract and the women at 38 weeks of gestation were excluded.
Institutional Ethical Committee (IEC) clearance was obtained for this study. A total of 637 antenatal women with asymptomatic bacteriuria were screened during the study period. From all the study population informed consent was received. The patients were provided with sterile universal containers for sample collection. They were instructed to collect their clean catch mid-stream urine during the outpatient consulting hours [14,15].
Methodology: Using calibrated loop (measures 1.3mm diameter, delivering 1 μL) urine samples were inoculated into blood agar and Cysteine Lactose Electrolyte Deficient agar (CLED) medium and incubated overnight at 37°C. The samples with significant colony count (≥105 CFU/mL) were further processed for biochemical identifications and antibiotic susceptibility testing. The antibiogram of the organisms to the routinely used antibiotics such as amikacin, cefotaxime, cefoperazone/sulbactam, colistin, cotrimoxazole, gentamycin, imipenem, meropenem, nalidixic acid, nitrofurantoin, norfloxacin and polymyxin B was determined by Kirby–Bauer disk diffusion test [16]. Gram-negative isolates which are resistant/intermediate to third generation cephalosporins were further tested for ESBL production.
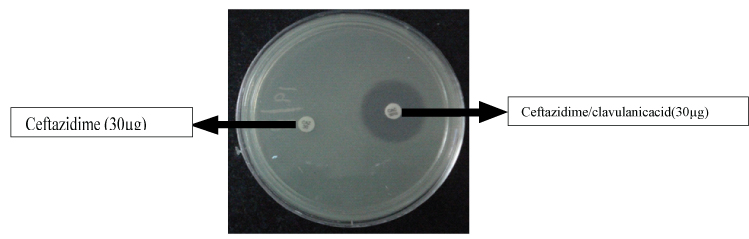
Phenotypic ESBL Detection: Phenotypic detection of ESBL producers was carried out using Ceftazidime 30 μg (CAZ) and ceftazidime with clavulanic acid 10 μg (CAC) disks. The Mueller-Hinton agar plates were inoculated and a disk of ceftazidime 30 μg (CAZ) and ceftazidime with clavulanic acid 10 μg (CAC) were placed 25 mm apart [Table/Fig-1]. The plates were incubated aerobically at 37°C. Isolates showing significant increase (≥5 mm) in a zone diameter in clavulanic acid combination versus ceftazidime alone were confirmed to be ESBL producers [16-18]. K. pneumoniae ATCC® 700603 TM (positive control) and E. coli ATCC® 25922 TM (negative control) were used for quality control [16].
Phenotypic assay for assessing the production of ESBL.
Isolates showing significant increase ≥ 5mm in a zone diameter in clavulanic acid combination versus ceftazidime alone were confirmed to be ESBL producers.(e.g, ceftazidime zone =6mm; Ceftazidime-clavulanic acid zone=15mm).
PCR and Sequencing of ESBL Amplicons: Phenotypically confirmed isolates with ESBL activity were screened by conventional PCR for the presence of cefotaxime hydrolyzing capabilities (CTX-M), sulfhydryl Variable (SHV) and Temoneira (TEM) encoding genes 2using gene specific primers mentioned in [Table/Fig-2] [18]. PCR conditions followed were 95°C for 5 minutes, 30 cycles with 95°C for 30 seconds, 55°C for 30 seconds, 72°C for 90 seconds and final extension at 72°C for 7 minutes. The amplicons were purified using Gene JET PCR clean up kit (Thermo Scientific, USA) and sent for sequencing to Macrogen Inc. Seoul, South Korea. The sequence chromatograms were checked and verified using Chromaslite 2.1. The sequences were analysed using BLAST and the allelic variants were identified [19].
List of primers used for screening and sequencing of ESBL encoding genes.
| S. No | β-lactamase gene | Primer name | Primer sequence (5’- 3’) | Amplicon sizes (bp) |
|---|
| 1. | blaCTX-M | CTX-M F CTX-M R | TCTTCCAGAATAAGGAATCCC CCGTTTCCGCTATTACAAAC | 909 |
| 2. | blaSHV | SHV F SHV R | TGGTTATGCGTTATATTCGCC GGTTAGCGTTGCCAGTGCT | 868 |
| 3. | blaTEM | TEM F TEM R | TCTTGGTCTGACAGTTACCAATGC TTGGTCTGACAGTTACCAATGC | 931 |
Results
A total of 101 (37%) ESBL producing GNB were isolated out of 271 Gram negative isolates, from 637 suspected asymptomatic bacteriuria antenatal mother’s mid-stream urine samples. Among these ESBL producers, 58% were Escherichia coli followed by Klebsiella pneumoniae (24%), Non Fermenting Gram Negative bacilli (NFGNB) (10%) and Citrobacter freundii (8%) as identified by phenotypic ESBL detection method. A larger proportion of the ESBL producers (73%) were found to be MDR isolates.
Resistogram analysis of these ESBL producing Gram-negative isolates by Kirby-Bauer disk diffusion method showed a wide range of resistance (74%-100%). Maximum resistance towards nitrofurantoin (100%) and nalidixic acid (86%) were observed among the NFGNB and E.coli isolates respectively. Antibiotic susceptibility/resistance pattern of these isolates against routinely used antibiotics were given in the [Table/Fig-3]. All these isolates showed very significant percentage of susceptibility towards carbapenems, Beta lactam/Beta lactamase Inhibitor (BL/BLI) combination, colistin and polymyxin B.
Antibiotic resistance pattern of ESBL positive Gram Negative Bacilli isolates.
| GEN* | COT | CTX | AK | IMP | MRP | CFS | PB | CL | NA | NX | NIT |
|---|
| E.coli (59) | 81% | 71% | 100% | 43% | 3% | 4% | 16% | 0% | 0% | 86% | 71% | 33% |
| K.pneumoniae (24) | 60% | 64% | 100% | 42% | 12% | 17% | 26% | 0% | 0% | 50% | 55% | 78% |
| C. freundii (8) | 87% | 87% | 100% | 75% | 0% | 0% | 0% | 0% | 0% | 37% | 75% | 62% |
| NFGNB (10) | 40% | 50% | 100% | 40% | 22% | 11% | 11% | 0% | 0% | 50% | 60% | 100% |
*GEN- Gentamycin, COT- Cotrimoxazole, CTX-Cefotaxime, AMK- Amikacin, IMP- Imipenem, MRP- Meropenem, CFS- Cefoperazone/Sulbactam, PB- Polymyxin B, CL- Colistin, NA- Nalidixic acid, NX- Norfloxacin, NIT- Nitrofurantoin, NFGNB- Non-fermentative gram-negative bacilli
PCR screening for the ESBL genes of all the phenotypic ESBL positive isolates confirmed that the incidence of blaCTX-M and blaSHV genes were 58%(59) and 4%(4), none of them showed amplification of blaTEM [Table/Fig-4 and 5]. The co-existence of blaCTX-M and blaSHV genes was observed among 19% (19) of the ESBL positive isolates. Among the E. coli ESBL producers, 67% (40) of them showed the presence of blaCTX-M and the co-production of blaCTX-M and blaSHV was observed only in 12%(7) of these isolates. In case of K. pneumoniae isolates, 33%(8) showed positive amplification for blaCTX-M and 50%(12) was found to carry both blaCTX-M and blaSHV. Fifty percent(5) of NFGNB carried blaCTX-M and 20% (2) showed blaSHV. Citrobacter freundii showed blaCTX-M alone in 75% (6) of isolates [Table/Fig-6]. Further, sequence analysis of blaCTX-M and blaSHV amplicons identified that all of them belong to a single allelic variant namely blaCTX-M-15 and blaSHV-1. The sequence variants of blaCTX-M and blaSHV genes identified from this study were submitted to Genbank and the accession numbers were obtained (MG989244-MG989270).
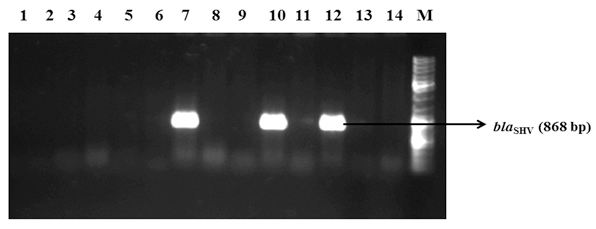
PCR amplification of blaSHV among the phenotypic ESBL positive isolates.
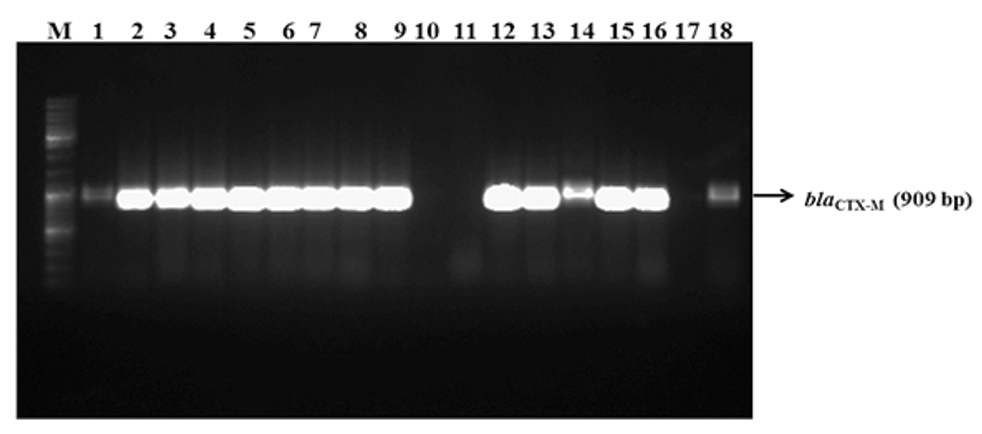
PCR amplification of blaCTX-M among the phenotypic ESBL positive isolates.
Distribution of β-lactamase genes among the ESBL producing isolates.
| Gene name | Escherichia coli (n=59) | Klebsiella pneumoniae (n=24) | NFGN B (10) | Citrobacterfreundii (8) | Total (n=101) |
|---|
| CTX-M | 40 (67%) | 8(33%) | 5(50%) | 6(75%) | 59 (58%) |
| SHV | 2(3%) | - | 2(20%) | - | 4 (4%) |
| TEM | - | - | - | - | - |
| CTX-M+SHV | 7(12%) | 12(50%) | - | - | 19 (19%) |
| TEM/TEM+CTX-M/TEM+SHV/TEM+SHV+CTX-M | - | - | - | - | - |
| None of the three genes | 10(18%) | 4(17%) | 3(30%) | 2(25%) | 19 (19%) |
| Total | 59 | 24 | 10 | 8 | 101 |
Discussion
Asymptomatic Bacteriuria (ASB) is very frequently observed among the pregnant woman and may lead to complications if left untreated [8]. The more severe and most common complications such as low birth weight, stillbirth, anaemia and sepsis during pregnancy necessitates the need to treat ASB at the earliest [9]. MDR is defined as non-susceptibility to at least one agent in three or more antimicrobial categories [20]. ASB with MDR isolates further complicates the outcomes during the course of treatment [21]. This study was carried out to ascertain the prevalence of MDR isolates among the ASB pregnant woman in a tertiary care hospital at Puducherry, India. Among the isolates collected from ASB pregnant women, the incidence of ESBL production occupies very significant predominance (37%). Alarmingly, 73% of them were multidrug resistant isolates. The overall reported incidence of ESBL in GNB ranges from 6 to 87% in India [9].
Persistence of MDR pathogens among the community and healthcare settings might be because of various factors like, antibiotic practicing policy, type of infections, nature of diseases, duration of hospital stay, level of aseptic procedures, environment, history of antibiotic usage and others. As a result of it, the prevalence of MDR pathogens have peaked drastically nowadays thus creating a substantial therapeutic burden to the infection control practices in almost all the health-care facilities, leading to marked increase in the morbidity and mortality.
Extensive usage of third generation cephalosporins and aztreonam was proven to be the cause for the emergence of resistant isolates carrying TEM and SHV β-lactamase encoding genes [22]. The overuse of ceftriaxone and horizontal transfer events were found to be the driving factor for the dissemination of CTX-M type of ESBL among the community [23]. Co-existence of all the three genes would lead to higher manifold of resistance to currently available drugs and their wide dissemination is of great concern for empirical management [24]. Unfortunately, together with their occurrence, if impermeability also accompanies, it may lead to even carbapenem resistance, which could be highly troublesome sign [25]. Though the number of molecular studies from India reporting the prevalence of ESBL producing K. pneumoniae and E.coli are scanty, a recent investigation from northern part of India reported maximum ESBL production among K. pneumoniae (52%), followed by E.coli (46%) [26]. These observations are not matching with our results as we found E. coli to be the predominant ESBL producer. And in contrast to our findings, E.coli and Klebsiella spp. exhibited comparatively less resistance to amikacin and nitrofurantoin (14% and 18% in E.coli, 43% and 34% in K. pneumoniae) [26]. One recent study by Manoharan A et al., reported susceptibility of the isolates of the family Enterobacteriaceae towards imipenem (100%), meropenem (100%), amikacin (90%) and piperacillin/tazobactam (85%) which was almost similar to our results [24]. Similar to the above observations, all our isolates showed significantly less resistance towards carbapenems, BL-BLI combinations and monobactam.
The prevalence of ESBL encoding genes varies based on the study population and region. Among various ESBL class β-lactamase genes, blaCTX-M, blaSHV and blaTEM were found to be more common in India [27]. In our study, the sequence analysis of ESBL genes revealed predominant presence of blaCTX-M-15 (58%) followed by blaSHV-1 (4%). Interestingly, blaTEM was completely absent and some of the isolates had combination of both blaCTX-M-15 and blaSHV-1 (19%). Studies from various parts of the globe showed varied predominance of genes among the ESBL producers. A study from Korea reported the presence of blaCTX-M-15 gene only in 6.7% of isolates out of 163 ESBL producers [28]. Though their percentage was very less, the existence of other blaCTX-M variants such as blaCTX-M-3, blaCTX-M-9 and blaCTX-M-14 was witnessed [28]. But, in our investigation only blaCTX-M-15 was detected, which is very significant finding. This suggests that a single variant is predominantly disseminating in our community. A study on ESBL producers among Lebanese population has revealed the presence of blaCTX-M-15 (80%), blaSHV (31%) and blaTEM (68%) reflecting the global dissemination that includes India [29]. Shahid M et al., from India reported the presence of blaCTX-M (29%) and blaSHV (14%) among the isolates of Enterobacteriaceae isolated from urine [30]. Though we couldn’t find the presence of blaTEM from our samples, a study conducted from central India reported blaTEM as the predominant ESBL encoding gene (48.7%), followed by blaCTX-M (7.6%) and blaSHV (5.1%) [31].
The individual incidence of blaCTX-M among our E. coli and K. pneumoniae isolates are in the order of 67% and 33% respectively. However, blaSHV was present only in E. coli (3%) and absent in K. pneumoniae. A study from Sudan described blaCTX-M to be present in 71% of E. coli and 68% of Klebsiella spp. isolates, wherein blaSHV was seen in 6% of E. coli and 63% of Klebsiella spp [32]. Though few of our E. coli isolates carried blaSHV, complete absence of blaSHV in Klebsiella spp. indicates their inability of quick dissemination across the species, when compared to blaCTX-M in our region. Contradictorily, Chandra V and Goswami M, from Gujarat, India reported 44% E. coli with blaTEM gene and 4% with both blaTEM and blaSHV genes [33]. Among their Klebsiella spp. isolates, 76% were found to carry both blaSHV and blaTEM genes, 20% and 4% showed amplification for blaSHV and blaTEM genes alone respectively [33].
When compared to all the previous reports from India, we found a steep increase in the percentage of blaCTX-M and blaSHV, which indicates the dissemination of these genes are increasing exponentially in the recent years [30,31]. Although blaTEM is predominant in central part of India, we couldn’t get any isolate to be positive for this gene. This is a noteworthy observation which is unexplainable. Fecal carriage of these organisms might be the major reason for wide dissemination and increased prevalence of ESBL producers poses a great risk for efficient treatment. As a result of improper treatment the complications associated with ASB results in increased morbidity and mortality. Lastly, blaCTX-M producing E.coli is in high predominance resulting in bacteriuria, which leads to significant mortality that is of great concern [34].
Limitation
Sample size was small to emphasis more on the findings due to the short duration of study period aimed. Due to the limited resource availability and financial constraints, this study was focused only on blaCTX-M, blaTEM and blaSHV/ESBL genes. Moreover, this study was carried out using samples collected from a single hospital and it may not reflect the overall global scenario.
Conclusion
With adequate and complete periodic antenatal check-up during all the three trimesters will effectively decrease the complications due to ASB. The incidence of MDR and ESBL producing isolates is a challenge to the clinician to manage and monitor the safe confinement during pregnancy. The ESBL incidence and different types of ESBL gene distribution will directly reflect the prevalence of these resistant genes among the community. Thus, it sounds that, there is an urgent need for the formulation of regional antimicrobial practicing policy by the stakeholders to implement and to monitor, so that some of the currently available antibiotics can be preserved for future long term needs.
*GEN- Gentamycin, COT- Cotrimoxazole, CTX-Cefotaxime, AMK- Amikacin, IMP- Imipenem, MRP- Meropenem, CFS- Cefoperazone/Sulbactam, PB- Polymyxin B, CL- Colistin, NA- Nalidixic acid, NX- Norfloxacin, NIT- Nitrofurantoin, NFGNB- Non-fermentative gram-negative bacilli
[1]. Global Antibiotic Resistance Partnership (GARP). Rationalizing antibiotic use to limit antibiotic resistance in IndiaIndian J Med Res 2011 134(4):577 [Google Scholar]
[2]. Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, Global trends in antimicrobial use in food animalsProc Natl Acad Sci U S A 2015 112(18):5649-54.10.1073/pnas.150314111225792457 [Google Scholar] [CrossRef] [PubMed]
[3]. Bhatt P, Tandel K, Shete V, Rathi KR, Burden of extensively drug-resistant and pandrug-resistant Gram-negative bacteria at a tertiary-care centreNew Microbes New Infect 2015 8:166-70.27257498 [Google Scholar] [CrossRef]
[4]. Kass EH, The role of asymptomatic bacteriuria in the pathogenesis of pyelonephritis. In: Quinn EL, Kass EH, editors. Biology of Pyelonephritis 1960 BostonLittle, Brown and Co:399-412. [Google Scholar]
[5]. Kincaid-Smith P, Bullen M, Bacteriuria in pregnancyLancet 1965 1:395-99.10.1016/S0140-6736(65)90001-2/10.1016/S0140-6736(65)90001-2 [Google Scholar] [CrossRef] [PubMed]
[6]. Babypadmini S, Appalaraju B, Extended spectrum β-lactamases in urinary isolates of Escherichia coli and Klebsiella pneumoniae-prevalence and susceptibility pattern in a tertiary care hospitalIndian J Med Microbiol 2004 22(3):172-74. [Google Scholar]
[7]. Jeyabalan A, Lain KY, Anatomic and functional changes of the upper urinary tract during pregnancyUrol Clin North Am 2007 34:01-06.10.1016/j.ucl.2006.10.00817145354 [Google Scholar] [CrossRef] [PubMed]
[8]. Zhanel GG, Harding GK, Guay DR, Asymptomatic bacteriuria. Which patients should be treated?Arch Intern Med 1990 150:1386-96.10.1001/archinte.1990.003901900550072196024 [Google Scholar] [CrossRef] [PubMed]
[9]. Stenqvist K, Dahlen-Nilsson I, Lidin-Janson G, Lincoln K, Oden A, Rignell S, Bacteriuria in pregnancy. Frequency and risk of acquisitionAm J Epidemiol 1989 129:372-79.10.1093/oxfordjournals.aje.a1151402912046 [Google Scholar] [CrossRef] [PubMed]
[10]. Hsu CD, Witler FR, Urogenital infection in preeclampsiaInternat J Gynaecol Obstet 1995 49(3):271-75.10.1016/0020-7292(95)02373-K [Google Scholar] [CrossRef]
[11]. Cohlan SQ, Cohlan SQ, Cohlan SQ, Cohlan SQ, National center for disease control. National treatment guidelines for antimicrobial use in infectious diseasesChapter Obstetrics and Gynecological infections 2016 V 1.0:18-29. [Google Scholar]
[12]. Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Changes in pathogens causing early-onset sepsis in very-low-birth-weight infantsN Engl J Med 2002 347:240-47.10.1056/NEJMoa01265712140299 [Google Scholar] [CrossRef] [PubMed]
[13]. Balla SR, Nemani S, Porter A, Rao BN, A prospective study of asymptomatic bacteriuria in pregnant women in a teaching hospital of semi urban setupInternational Journal of Scientific Research 2016 5(11):76-78. [Google Scholar]
[14]. Millar LK, Cox SM, Urinary tract infections complicating pregnancyInfect Dis Clin North AM 1997 11:13-26.10.1016/S0891-5520(05)70339-1 [Google Scholar] [CrossRef]
[15]. Imade PE, Izekor PE, Eghafona NO, Enabulele OI, Ophori E, Asymptomatic bacteriuria among pregnant womenN Am J Med Sci 2010 2(6):263-66. [Google Scholar]
[16]. CLSIPerformance Standards for Antimicrobial Susceptibility Testing; CLSI Document M02 2011 13th EditionWayne, PAClinical and Laboratory Standards Institute [Google Scholar]
[17]. Kumar MS, Lakshmi V, Rajagopalan R, Occurrence of extended spectrum beta-lactamases among Enterobacteriaceae spp. isolated at a tertiary care instituteIndian J Med Microbiol 2006 24(3):208-11. [Google Scholar]
[18]. Kiratisin P, Apisarnthanarak A, Laesripa C, Saifon P, Molecular characterization and epidemiology of extended-spectrum-beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates causing health care-associated infection in Thailand, where the CTX-M family is endemicAntimicrob Agents Chemother 2008 52(8):2818-24.10.1128/AAC.00171-0818505851 [Google Scholar] [CrossRef] [PubMed]
[19]. http://technelysium.com.au/wp/chromas/ [Google Scholar]
[20]. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistanceClin Microbiol Inect 2012 18(3):268-81.10.1111/j.1469-0691.2011.03570.x21793988 [Google Scholar] [CrossRef] [PubMed]
[21]. Goudarzi H, Douraghi M, Ghalavand Z, Goudarzi M, Assessment of antibiotic resistance pattern in Acinetobacter baumannii carrying blaoxa type genes isolated from hospitalized patientsNovelty in Biomedicine 2013 1(2):54-61. [Google Scholar]
[22]. Paterson DL, Bonomo RA, Extended-spectrum beta-lactamases: a clinical updateClin Microbiol Rev 2005 18(4):657-86.10.1128/CMR.18.4.657-686.200516223952 [Google Scholar] [CrossRef] [PubMed]
[23]. Cavaco LM, Abatih E, Aarestrup FM, Guardabassi L, Selection and Persistence of CTX-M producing E.coli in the intestinal flora of pigs treated with Amoxicilin, ceftriofur or cefquinomeAntimicrob Agents Chemother 2008 52(10):3612-16.10.1128/AAC.00354-0818644956 [Google Scholar] [CrossRef] [PubMed]
[24]. Manoharan A, Premalatha K, Chatterjee S, Mathai D, Correlation of TEM, SHV and CTX-M extended spectrum beta-lactamases among Enterobacteriaceae with there in vitro antimicrobial susceptibilityIndian J Med Microbiol 2011 29(2):161-64.10.4103/0255-0857.8179921654112 [Google Scholar] [CrossRef] [PubMed]
[25]. Shah PM, Isaacs RD, Etrapenem, the first of a new group of carbapenemsJ Antimicrob Chemother 2003 52:538-42.10.1093/jac/dkg40412951340 [Google Scholar] [CrossRef] [PubMed]
[26]. Kaur M, Aggarwal A, Occurrence of the CTX-M, SHV and the TEM Genes among the extended spectrum β-lactamase producing isolates of enterobacteriaceae in a tertiary care hospital of north IndiaJ Clin Diagn Res 2013 7(4):642-45.10.7860/JCDR/2013/5081.287223730637 [Google Scholar] [CrossRef] [PubMed]
[27]. Mathai D, Manoharan A, Vasanthan G, Epidemiology and implications of ESBLCrit Care Update 2009 14:152-62. [Google Scholar]
[28]. Kim J, Lim YM, Jeong YS, Seol SY, Occurrence of CTX-M-3, CTX-M-15, CTXM- 14, and CTX-M-9 extended-spectrum beta-lactamases in Enterobacteriaceae clinical isolates in KoreaAntimicrob Agents Chemother 2005 49(4):1572-75.10.1128/AAC.49.4.1572-1575.200515793142 [Google Scholar] [CrossRef] [PubMed]
[29]. Daoud Z, Salem Sokhn E, Masri K, Matar GM, Doron S, Escherichia coli Isolated from Urinary Tract Infections of Lebanese Patients between 2005 and 2012: Epidemiology and Profiles of ResistanceFront Med (Lausanne) 2015 2:2610.3389/fmed.2015.00026 [Google Scholar] [CrossRef]
[30]. Shahid M, Singh A, Sobia F, Rashid M, Malik A, Shukla I, bla(CTX-M), bla(TEM), and bla(SHV) in Enterobacteriaceae from North-Indian tertiary hospital: high occurrence of combination genesAsian Pac J Trop Med 2011 4(2):101-05.10.1016/S1995-7645(11)60046-1 [Google Scholar] [CrossRef]
[31]. Bajapi T, Pandey M, Varma M, Bhatambare GS, Prevalence of TEM, SHV and CTX-M Beta-Lactamase genes in the Urinary isolates of a tertiary care hospital.Avicenna J M 2017 7(1):12-16.10.4103/2231-0770.19750828182026 [Google Scholar] [CrossRef] [PubMed]
[32]. Ahmed AB, Omar AO, Asghar AH, Elhassan MM, Prevalence of TEM, SHV and CTX-M genes in Escherichia coli and Klebsiella spp. urinary isolates from Sudan with confirmed ESBL phenotypeLife Sci J 2013 10:191-95. [Google Scholar]
[33]. Chandra V, Goswami PN, Detection of TEM & SHV genes in extended spectrum beta-lactamase (ESBL) producing E.coli & Klebsiella pneumoniae isolated from a tertiary care cancer hospitalNational journal of Medical research 2014 4(3):201-04. [Google Scholar]
[34]. Tumbarello M, Sanguinetti M, Montuori E, Trecarichi EM, Posteraro B, Fiori B, Predictors of mortality in patients with blood stream infections caused by ESBL producing Enterobactercae: importance of inadequate initial antimicrobial treatmentAntimicrob Agents Chemother 2007 51:987-94.10.1128/AAC.01509-0617387156 [Google Scholar] [CrossRef] [PubMed]