As much as pain is a protective mechanism, it’s perhaps the most feared symptom of a disease. Pain in children compared to adults has been essentially an ignored dimension of care. Postoperative pain in children can be emotionally very distressing for those concerned, particularly the parents and the anaesthesiologist involved. This is even more relevant today as anaesthesiologist takes pride in being called the perioperative physician who can relieve pain. There are so many confounding factors such as thirst, hunger, stranger anxiety or even postoperative delirium which can confuse the postoperative pain assessment in a child [1].
Caudal analgesia or anaesthesia is the most popular central neuraxial technique in infra umbilical surgeries in paediatric age group. Caudal block is reasonably safe, easy to perform and gives reliable results. Most common drugs used in caudal block include local anaesthetics and opioids. A single shot caudal injection with local anaesthetic provides analgesia for limited duration. Use of adjuvants to prolong the duration of the block is a common practice [2]. Addition of opioids, Adrenaline, Ketamine, Midazolam or Dexmedetomidine can prolong duration of action of local anaesthetics. Each of these agents may have its limitations or disadvantages too. Neostigmine is another agent tried as adjuvant to local anaesthetic agents in caudal block with reports of prolonged duration of analgesia [3]. Use of caudal Neostigmine has been limited by unacceptably high incidence of nausea and vomiting [4-6]. Though it is an off label use, safety of Neostigmine in caudal use has been demonstrated in many studies [3-8]. This study was first of its kind in our hospital, which is a tertiary care centre in Southern India. Many additives like Midazolam and Dexmedetomidine have been previously evaluated at our institution as additives to local anaesthetic drugs in caudal anaesthesia, but Neostigmine was being evaluated for the first time. Although previous studies tried to evaluate the efficacy of Caudal Neostigmine at varying doses, the high incidence of nausea and vomiting made it unpopular [4-6]. One of the reasons for the high incidence of nausea and vomiting could be the higher dose (4 mcg/kg) used. We used the optimal dose of 2 mcg/kg of Caudal Neostigmine as no added benefit was demonstrated with the higher dose of 4 mcg/kg in the study by Karaaslan K et al., [3].
This double blinded prospective randomized controlled study was aimed to study the efficacy of Neostigmine (2 mcg/kg) as an adjuvant to 0.25% Bupivacaine 1 mL/kg in Caudal blocks in paediatric inguinal herniotomies.
Materials and Methods
This was a prospective, double blinded randomised clinical study. The study was carried out at Medical College, Trivandrum between March 2004 and May 2004 and was undertaken after obtaining the approval of Institutional review board and ethical committee. After getting written informed parental consent, 40 ASA1 children of either gender, aged 2-6 years, undergoing inguinal herniotomy were randomly allocated to two groups (n=20 each). The 1st group (Group B) received caudal injection of 0.25% Bupivacaine while the 2nd group (Group BN) received caudal injection of 0.25% Bupivacaine plus Neostigmine after induction of general anaesthesia. Sample size of 20 subjects per group was chosen to obtain a statistical significance assuming an alpha error of 0.05 and power of 0.9. Hence 40 children were included in the study with randomisation to two groups of 20 children each. Randomisation was done using sealed envelope technique. The anaesthesiologists involved were blinded to the study. The anaesthesiologist preparing and blinding the drugs were not involved with the conduct of the study. Double blindedness was ensured by involving different anaesthesiologists, one person to prepare and hand over the study drug, a 2nd person to do the caudal block and another person to do the observations. The anaesthesiologists administering the caudal block and making the observations were blinded to the study drugs.
Exclusion criteria were any contraindications to caudal block, coagulopathies, recent respiratory tract infections, bronchial asthma, and allergy to any of the study drugs or any pre existing neurological or spinal disease.
Routine pre anaesthetic check up done and written informed parental consent was obtained. Age and weight were noted. Basal heart rate and respiratory rate were noted. Milk and solids were restricted after midnight, but clear fluids allowed upto two hours prior to anaesthetic induction. Premedication was with Syp Pedicloryl 75 mg/kg given one hour prior to surgery. All drugs were prepared before patient was brought to operating room. Anaesthesia machine check was done and all essential airway equipments including working laryngoscopes, appropriate sized LMA, endotracheal tubes and oropharyngeal airways were kept ready.
Gas induction was done with Sevoflurane in Oxygen and Nitrous oxide. By engaging in constant conversation and 0.5% increase in inspired concentration of Sevoflurane every 3rd to 4th breath, smooth transition to General Anaesthesia was produced. Monitoring included continuous ECG, Pulse oximetry, Non Invasive Blood Pressure and End Tidal CO2. A 22 G cannula was inserted on the dorsum of hand and Ringers Lactate was started and administered according to calculated requirements. IV Propofol 1-1.5 mg/kg was given and once adequate depth of anaesthesia was ensured, appropriate sized LMA was inserted and proper positioning was confirmed. Mapleson F was the breathing system used. Anaesthesia was maintained with 1.5-2% Sevoflurane and 66% Nitrous oxide in Oxygen with the patient on spontaneous ventilation as early as it returned. Fresh gas flow was kept at twice the minute ventilation.
Caudal block: Child was placed in lateral position with operative site down and legs flexed at hip and knee. Adequacy of airway was ensured throughout. The back was disinfected using Povidone Iodine and then draped. Under strict aseptic precautions, Caudal block was instituted using a 23 G scalp vein needle with a short bevel. The needle position was confirmed with loss of resistance technique by injection of 1 mL of saline. After negative aspiration for blood or CSF, the drug was injected at rate of 0.2 mL/sec in 2 mL increments. The 1st group (Group B) received caudal injection of 0.25% Bupivacaine 1 mL/kg plus 1mL Normal Saline 0.9% after induction of general anaesthesia. The 2nd group (Group BN) received caudal injection of 0.25% Bupivacaine 1 mL/kg plus Neostigmine 2 mcg/kg diluted with Normal Saline 0.9% made up to volume of 1 mL, after administration of general anaesthesia. The strength of Neostigmine preparation, commercially available in ampoule form is 500 mcg/mL. The time of injection was noted and the duration of analgesia was the time taken from the time of injection of the drug to the timing of 1st rescue analgesia. After the injection, child was positioned supine. No other analgesics were given by any route preoperatively or intraoperatively. Patients were randomly allocated to two groups (n=20 each). Group B received 1 mL/kg of 0.25% Bupivacaine plus 1 mL 0.9% Normal Saline. Group BN received 0.25% Bupivacaine 1 mL/kg plus 1 mL 0.9% Normal Saline with Neostigmine (Neostigmine 2 mcg/kg diluted with Normal Saline 0.9% made up to volume of 1 mL). Maximum volume administered was 20 mL.
At the end of surgery, anaesthetic agents were discontinued and 100% Oxygen was given for 2 minutes. Then LMA was removed and airway maintained with mask. After ensuring that the vital signs were stable, child was shifted to recovery room in left lateral position. The time from discontinuation of anaesthetic to spontaneous eye opening was noted. The child was later discharged to the post operative ward after recovery room discharge criteria were met. The AIIMS pain discomfort scale [9] [Table/Fig-1] was used for assessment in our study and was done for a period upto 24 hours following caudal block. The degree of pain relief was assessed at 1, 2, 4, 8, 12 and 24 hours after Caudal block.
Pain discomfort scale (AIIMS).
| Parameters | Criteria | Points |
|---|
| 1. Respiratory rate | + <20% of preoperative baseline+20-50% of preoperative baseline+ >50% of preoperative baseline | 012 |
| 2. Heart rate | +10% of preoperative baseline+20% of preoperative baseline+ 30% of preoperative baseline | 012 |
| 3. Discomfort | CalmRestlessAgitated | 012 |
| 4. Crying | No cry or cry responding to water, food or parental presenceCry responding to tender loving careCry not responding to tender loving care | 012 |
| 5. Pain at site of operation | No painStates pain vaguelyCan localise pain | 012 |
Supplementary analgesia using oral paracetamol 15 mg/kg was given to patients who had a pain score equal to or more than 4 at any time during the first 24 hours. The time from discontinuation of anaesthetic to spontaneous eye opening was noted. Duration of motor block was assessed by determining when the child began to move legs. Time to first micturition was noted. Frequency of nausea and vomiting was also noted. Patients were discharged 24 hours after the surgical procedure.
Statistical Analysis
The data collected were entered in the master chart and statistical constants were computed to get valid inference about the data. The hypothesis formulated was tested by student’s t-test in case of quantitative data.
Results
Age and weight distribution: Both groups were identical when patient demographics were analysed. Mean age in group B was 3.83 years, while in Group BN it was 3.88 years. Mean weight was 13.16 kg in Group B while it was 13.84 kg in Group BN. These differences were not statistically significant.
The mean duration of surgery was 18 minutes in Group B and 20 minutes in Group BN.
Pain scores at various time intervals were noted and tabulated. Superior analgesia was noted in group BN compared to group B [Table/Fig-2].
Comparative pain scores at various time intervals.
| Monitoring time(in hours) | Pain scoresGroup B | Pain scoresGroup BN |
|---|
| 1 | 0 | 0 |
| 2 | 0.16±0.37 | 0 |
| 4 | 0.76±0.83 | 0.04±0.2 |
| 8 | 2.6±1.2 | 0.72±0.84 |
| 12 | 3.52±0.87 | 2.2±1.04 |
| 24 | 3.44±0.91 | 3.32±1.14 |
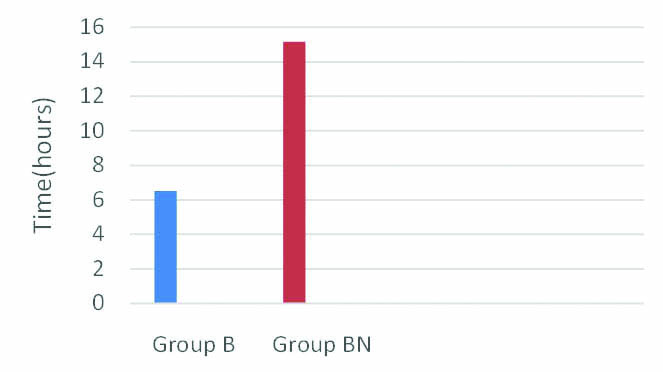
The mean time taken to first analgesia was 6.52 hours in Group B compared to 15.16 hours in Group BN [Table/Fig-3]. The large difference was highly significant according to t-test. A p-value less than 0.001 was obtained.
Comparison of mean time to first analgesia in both groups (in hours).
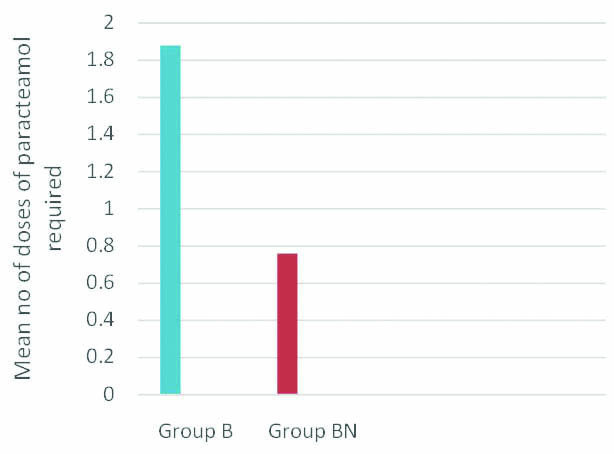
Mean value of number of doses of paracetamol required was 1.88 in Group B compared to 0.76 in Group BN. p-value was less than 0.001 and was statistically significant. Decreased analgesic requirement was noted in group BN [Table/Fig-4].
Comparison of analgesic requirements of both groups (No. of doses of Paracetamol required).
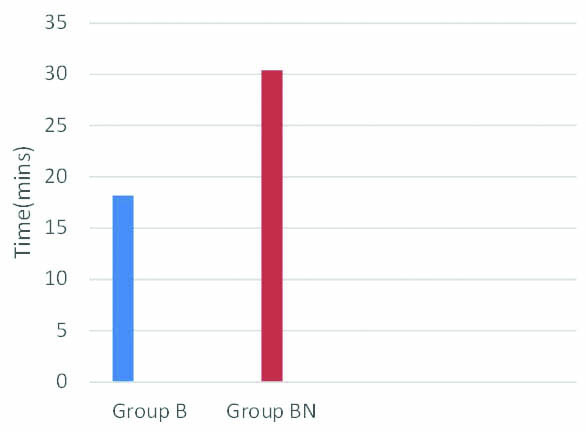
Mean time to spontaneous eye opening was 18.2 minutes in Group B while it was 30.4 minutes in group BN [Table/Fig-5]. p-value was less than 0.001 and this was statistically significant.
Comparison of time to spontaneous eye opening (in mins).
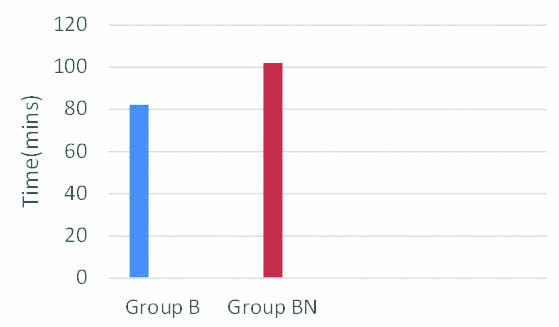
Time taken to spontaneous leg movements after surgery was assessed and was found to be 82.2 minutes in Group B compared to 102 minutes in Group BN [Table/Fig-6]. p-value was less than 0.001 and was statistically significant.
Comparison of time to spontaneous leg movements (in mins).
Time taken for micturition after caudal block in Group B was 4.01 hours while it was 4.70 hours in Group BN. p-value was greater than 0.05, no statistical significance was noted between the 2 groups.
Surprisingly, no incidence of nausea and vomiting were observed in any study subjects in both the groups. Hence no statistical test was done. Similar result of zero incidence of nausea and vomiting after caudal Neostigmine was reported by Gupta et al., [10]. Other complications such as respiratory depression and hypotension were not observed in any of the subjects in the study
Discussion
Adjuncts are added to caudal block to prolong the duration of action. In our study, the addition of Neostigmine 2mcg/kg as adjuvant to 0.25% Bupivacaine 1 mL/kg, resulted in significant prolongation of analgesia with reduced requirements in postoperative analgesia. Analgesic effect may be explained by transdural diffusion of Neostigmine into cerebrospinal fluid. Neostigmine, an anticholinesterase drug, inhibits breakdown of acetylcholine and increases acetylcholine levels at the dorsal horn of spinal cord. Spinal muscarinic receptors play the important role in analgesia of spinal or epidural neostigmine [11]. Despite the analgesic efficacy of this adjuvant, the side effects of neuraxial Neostigmine such as nausea and vomiting has not made it very popular [11]. Another reason could be the off label use and the potential fear of neurotoxicity by the preservatives. As the use of prophylactic antiemetics has been advocated in the perioperative setting, we could argue that there is no harm in giving antiemetics preoperatively where caudal block is instituted [12,13]. Ondansetron, given towards the end of surgery at a dose of 0.1 mg/kg probably resulted in zero incidence of nausea and vomiting in our study [11,14]. There will be concern about “off label” use of anaesthetic drugs, but prescription of drugs for off label uses is an acceptable practice, since the decision to use that drug is based on “good medical practice” [15]. By referring to peer reviewed information from high quality medical journals on use of anaesthetic drugs, anaesthesiologists may be able to practice evidence based medicine rather than just restricting their practice to information given in manufacturer’s package insert [16,17]. Although there was statistically significant postoperative sedation and delay in return of motor function in Group BN compared to Group B, these side effects weren’t serious ones taking into consideration the fact that there was significant prolongation of the analgesic effect.
Limitation
In this era of evidence based medicine, practice based on clinical evidence and peer reviewed information can be innovative. Despite this, concerns about “off label” use of drugs like Neostigmine still exist. The small number of patients in our study could be yet another limiting factor.
Conclusion
Addition of Neostigmine 2 mcg/kg to 0.25% Bupivacaine for caudal anaesthesia prolongs the duration of postoperative analgesia and reduces the requirement for postoperative analgesics significantly. Administration of prophylactic antiemetic, Ondansetron, when given towards the end of surgery, has been quite useful to negate the incidence of postoperative nausea and vomiting.