Diabetic Peripheral Neuropathy is a non-inflammatory disease process associated with diabetes mellitus characterised by sensory and/or motor disturbances in the peripheral nervous system. It is associated with long term complications affecting various organs especially eyes, kidneys, nerves, blood vessels and heart. It primarily affects the micro vascular circulation in the extremities; ‘sugar coated capillaries’ limits the blood supply to the superficial and deep structures. DPN further leads to infections, increasing the risk of foot ulcers and non-traumatic amputations. One extremely dreadful and morbid complication associated with diabetes is the foot complication which can range from an ulcer to any extent of amputation [1].
There are 387 million people with diabetes in the world and 75 million people in the Southeast Asia (SEA) region, and by 2036 this will rise to 123 million [2]. In 40-50% of people with diabetes mellitus Type I or Type II, neuropathy develops within 10 years of the onset. The estimated prevalence of diabetic neuropathy among diabetic patients is 30% in hospital patients and 20% in the community [3]. There is an increased incidence of foot ulceration and non-traumatic amputations among patients with DPN. The characteristic features of DPN are the absence of sensations and are clinically present in patients at various forms. It is estimated that 60% of diabetic foot is neuropathic in origin, 30% are ischaemic and 20% are infectious [1].
Epidemiological data indicate that the prevalence of diabetic neuropathy is higher in Type II than Type I diabetes [4]. Studies evinced that DPN is a key element in the casual pathway of foot ulceration and other lower extremity complications. The undiagnosed DPN complications lead to impaired quality of life and increase in mortality rate. This emphasises on early detection to optimise effective risk management including adequate foot care, patient education and future pharmacological therapy [5].
Vibration perception threshold plays a pivotal role in early detection of DPN and subsequently it reduces the risk for foot ulceration. In VPT, the electric current mode is converted into vibration mode and transverse to the patient. VPT measures large nerve fiber integrity and perception is normally poorer in lower extremity than the upper extremity. The VPT values are graded as mild (15-20v), moderate (20-25v), and severe (>25v). It may extent up to 50v.
Attempt to predict the development of diabetic neuropathic foot ulcer using VPT values indicate that VPT<15v had a cumulative incidence of foot ulceration of 2.9% compared with 19.8% in patients with a VPT>25v. VPT is an effective predictor of the risk of foot ulceration in diabetes and therefore could be used to target for early identification and treatment [6].
Materials and Methods
Design, sample and sampling: Descriptive survey was conducted among 220 diabetic patients attending the Department of Podiatry in a selected Tertiary Care hospital in Southern India from December 2015 to March 2016. The study subjects were selected by purposive sampling technique. The subjects were the diabetic patients who attended the Podiatry clinic for VPT measurement. Those who were reported or diagnosed with cognitive impairment were excluded from the study.
Data collection tools: Data were collected using structured questionnaire on clinical symptoms and S-LANSS pain score scale to assess the neuropathic pain, which is a standardised tool [7]. The S-Leeds Assessment of Neuropathic Symptoms and Signs Pain Scale (LANSS) pain score scale is a self-report version of the LANSS. The S-LANSS aims to identify pain of predominantly neuropathic origin, as distinct from nociceptive pain, without the need for clinical examination. The VPT value were graded as normal (<15v), mild (15-20v), moderate (20-25v) and <25 as severe neuropathy. The S-LANSS pain score scale had a sensitivity of 74% and specificity of 89%. Before conducting the main study, a pilot study was conducted among 22 patients to assess the feasibility of the study and the study was found to be feasible.
Data collection procedure: After obtaining formal ethical clearance from the Ethical Committee of the Institution, subjects were selected using purposive sampling and informed consent was also obtained from each of the subjects before starting the data collection. The data collection tools were administered to assess the demographic data, clinical symptoms and neuropathic pain.
Statistical Analysis
Data were analysed using both descriptive and inferential statistics. Descriptive statistics-frequency and percentage were used to analyse the socio demographic data and express the S-LANSS pain score. Fisher’s Exact test was used to find the association between VPT and diabetic peripheral neuropathy score. Pearson correlation was computed find the correlation between VPT score and number of symtoms.
Results
The data were analysed using frequency, percentage and association of VPT values and DPN was determined by Fisher’s-exact test.
Socio-demographic data: Out of the 220 patients, majority were above 40 years (212,96.36%) and females (136,61.81%). A small minority (11,5%) had the habit of smoking/chewing tobacco and consuming alcohol (36,16.36%).
Clinical data: Majority (150,68%) had diabetes for more than 10 years and in that 58 (26%) were diabetic for more than 20 years. Hypertension was the predominant co-morbidity (121,57.3%) followed by dislipidemia (101,45.9%). Lipid profile was not done by a few (89,40.5%) and out of the remaining 60%, 27 (20.6%) had >200 mg/dL of total cholesterol, 74 (56.49%) had more than 40 mg/dL of HDL and 59 (45.06%) had LDL above 100 mg/dL. Similarly, 57 (25.7%) had not tested their HbA1c level and 132 (60%) had 7% and above which is above the reference range of diabetics under treatment [8,9].
Clinical symptoms of diabetic peripheral neuropathy: The majority (122, 55.5%) had the autonomic symptom “scaly/dry skin” when both the legs were concerned, which was followed by “muscle cramp” (motor symptoms) in 109 (49.5%) subjects and the sensory symptom “pain” among 91 subjects (41.4%) [Table/Fig-1]. When individual legs were concerned, the most prominent symptom was pain {(31, 14%) in right leg/foot and 34 (15.4%) in left leg/foot}.
Clinical symptoms of diabetic peripheral neuropathy (n=220).
| Clinical Symptoms | Area |
|---|
| Right leg/foot | Left leg/foot | Both legs |
|---|
| f | % | f | % | f | % |
|---|
| Sensory Symptoms |
| Slipping of foot | 7 | 3.2 | 9 | 4.1 | 62 | 28.2 |
| Pain | 31 | 14.0 | 34 | 15.4 | 91 | 41.3 |
| Numbness | 16 | 7.3 | 19 | 8.6 | 7 | 3.18 |
| Pin and needle sensation in dorsal aspect | 5 | 2.3 | 6 | 2.7 | 46 | 20.9 |
| Pin and needle sensation in plantar aspect | 7 | 3.2 | 10 | 4.5 | 14 | 6.4 |
| Unusual colour with pain | 4 | 1.8 | 1 | 0.5 | 34 | 15.5 |
| Dip or sock foot in cold water | 2 | 0.9 | 2 | 0.9 | 11 | 5 |
| Sensation of bugs or worms crawling | 2 | 0.9 | 2 | 0.9 | 11 | 5 |
| Perception of walking on broken glass | 0 | 0 | 0 | 0 | 20 | 9.1 |
| Immersed in cold water/block of ice | 4 | 1.8 | 0 | 0 | 5 | 2.3 |
| Motor symptoms |
| Unsteadiness in walking | 10 | 4.5 | 15 | 6.8 | 71 | 32.2 |
| Muscle cramp | 5 | 2.3 | 6 | 2.7 | 109 | 49.5 |
| Oedema | 4 | 1.8 | 1 | 0.5 | 34 | 15.5 |
| Clawed toe | 3 | 1.4 | 2 | 0.9 | 4 | 1.8 |
| Charcot foot | 0 | 0 | 0 | 0 | 0 | 0 |
| Osteomylities | 9 | 4.1 | 8 | 3.6 | 1 | 0.5 |
| Deformed foot | 1 | 0.5 | 0 | 0 | 0 | 0 |
| Autonomic symptoms |
| Scaly/dry skin | 1 | 0.5 | 5 | 2.3 | 122 | 55.4 |
| Wound/ulcer | 25 | 11.3 | 21 | 9.5 | 4 | 1.8 |
| Itching over lower limb | 3 | 1.4 | 5 | 2.3 | 56 | 25.5 |
| Peeling/maceration | 5 | 2.3 | 9 | 4.1 | 30 | 13.6 |
Study result presented in [Table/Fig-2] shows that 219 out of 220 (99.5%) subjects had at least one symptom of DPN. The most striking symptom was sensory symptom, which was presented in mammoth proportion (199, 90.5%) followed by motor symptom (180, 81.8%) and autonomic symptom (166, 75.5%). It was a noteworthy finding that all these symptoms were presented in more than 70% of patients. The pain assessment using S-LANSS indicate that 47 (21.2%) had neuropathic pain.
Overall symptoms of diabetic peripheral neuropathy (n=220).
| Symptoms of DPN | Frequency (f) | Percentage (%) |
|---|
| Sensory symptoms | 199 | 90.5 |
| Motor symptoms | 180 | 81.8 |
| Autonomic symptoms | 166 | 75.5 |
| Dermatological symptoms | 154 | 70 |
| Symptomatic patients | 219 | 99.5 |
| Asymptomatic patients | 1 | 0.5 |
DPN: Diabetic peripheral neuropathy
Vibration perception threshold value: Normal VPT were observed only in 12 subjects (5.5%) and the remaining had neuropathy of varying degree with the majority (152, 69%) having severe neuropathy, followed by moderate neuropathy among 31 (14.1%) [Table/Fig-3].
Vibration perception threshold values (n=220).
| VPT values (V) | Frequency (f) | Percentage (%) |
|---|
| Normal (below 15 V) | 12 | 5.5 |
| Mild (15-20 V) | 25 | 11.4 |
| Moderate (20-25 V) | 31 | 14.1 |
| Severe (above 25 V) | 152 | 69.0 |
Association between Vibration Perception Threshold values and clinical symptoms: Data were collected regarding 28 symptoms of diabetic peripheral neuropathy and the association with vibration perception threshold value was computed using Fisher’s-exact test. VPT value was significantly associated with 11 clinical symptoms [Table/Fig-4]. VPT was significantly associated with the sensory symptoms like slipping of the chapels (p<0.001), numbness/tingling (p=0.009), pain or needle sensation, plantar (p<0.001); motor symptoms like muscle cramps (p<0.001), oedema (p<0.001), osteomyelitis (p=0.037); autonomic symptoms like scaly/dry skin (p=0.005), unhealed wound/ulcer (p<0.001) and dermatologic symptoms like callus (p<0.001), hyper pigment plaque/patches (p=0.005) and blackish discolouration of toes (p<0.001).
Association between VPT values and clinical symptom of diabetic peripheral neuropathy calculated using Fisher’s-exact test (n=220).
| Diabetic peripheral neuropathy symptoms | Category | Vibration perception threshold | Fisher’s value | p-value |
|---|
| Normal <15 V (12) | Mild 15-20 V (25) | Moderate 20-25 V (31) | Severe >25 V (152) |
|---|
| Sensory Symptoms |
| Slipping of the chapel (slippers) | Yes | 0 (0.0%) | 1 (4%) | 4 (12.9%) | 73 (48%) | 28.519 | 0.001 |
| No | 12 (100%) | 24 (96%) | 27 (87.1%) | 79 (52%) |
| Numbness/Tingling | Yes | 7 (58.3%) | 11 (44%) | 13 (41.9%) | 104 (68.4%) | 11.247 | 0.009 |
| No | 5 (41.7%) | 14 (56%) | 18 (58.1%) | 48 (31.6%) |
| Pin or needle sensation plantar | Yes | 0 (0.0%) | 0 (0%) | 4 (12.9%) | 44 (28.9%) | 18.041 | 0.001*** |
| No | 12 (100%) | 25 (100%) | 27 (87.1%) | 108 (71.1%) |
| Motor Symptoms |
| Muscle cramps | Yes | 1 (8.3%) | 9 (36%) | 13 (41.9%) | 97 (63.8%) | 21.349 | 0.001*** |
| No | 11 (91.7%) | 16 (64%) | 18 (58.1%) | 55 (36.2%) |
| Oedema | Yes | 2 (16.7%) | 6 (24%) | 6 (19.4%) | 101 (66.4%) | 40.407 | 0.001*** |
| No | 10 (83.3%) | 19 (79%) | 25 (80.6%) | 51 (33.6%) |
| Osteomyelitis | Yes | 0 (0%) | 0 (0%) | 0 (0%) | 8 (11.8%) | 7.461 | 0.037 |
| No | 12 (100%) | 25 (100%) | 31 (00%) | 134 (88.2%) |
| Autonomic symptom |
| Scaly/dry skin | Yes | 4 (33.3%) | 9 (36%) | 15 (48.4%) | 100 (65.8%) | 12.772 | 0.005*** |
| No | 8 (66.7%) | 16 (64%) | 16 (51.6%) | 52 (34.2%) |
| Unhealed wound/ulcer | Yes | 0 (0.0%) | 0 (0%) | 2 (6.5%) | 48 (31.6%) | 24.7222 | 0.001*** |
| No | 12 (100%) | 25 (100%) | 25 (93.5%) | 104 (68.4%) |
| Callus | Yes | 0 (0%) | 3 (12%) | 9 (29%) | 64 (42.1%) | 17.472 | 0.001*** |
| No | 12 (100%) | 22 (88%) | 22 (71%) | 88 (57.9%) |
| Hyperpigmented plaque/patches | Yes | 0 (0%) | 1 (4%) | 3 (9.7%) | 39 (25.7%) | 12.096 | 0.005*** |
| No | 12 (100%) | 24 (96%) | 28 (90.3%) | 113 (74.3%) |
| Blackish discolouration of toes | Yes | 0 (0.0%) | 0 (0%) | 2 (6.5%) | 63 (41.4%) | 38.743 | 0.001*** |
| No | 12 (100%) | 25 (100%) | 29 (93.5%) | 89 (58.6%) |
p<0.05, *** significant at <0.001
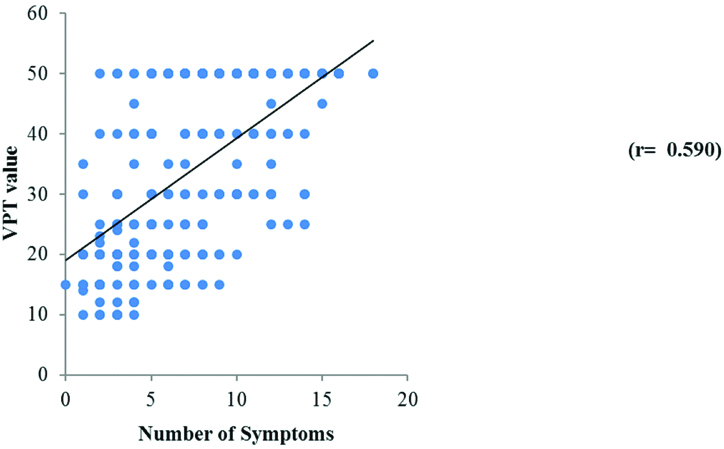
The correlation between the VPT value and the number of symptoms presented as scatter diagram shows a positive correlation (r=0.590) between the VPT score and the number of symptoms indicating that as the number of symptoms increases, the score of the vibration perception threshold also goes high [Table/Fig-5]. This in turn increases the risk for complications. All these suggest the role of VPT measurement as a key test for DPN.
Scatter plot to show the correlation between VPT score and number of symptoms.
Discussion
In the present study majority were females (136, 61.81%), above 40 years of age (212, 96.36%) and had diabetes for more than 10 years (150, 68%). Clinical symptoms of diabetic peripheral neuropathy of varying degree were reported in 219 (99.5%) out of 220 subjects.
Finding is supported by the study conducted to determine the factors associated with diabetic peripheral neuropathy and more particularly its relation to precisely assessed microangiopathy [10]. Study reports that the percentage of women with severe clinical neuropathy was significantly higher than that of men, and the clinical neurological stage. Clinical stage correlated only with gender and duration of diabetes. In the multivariate analysis, 17 parameters correlated with duration of diabetes, nine correlated with age, seven with glycemic control, and only one with gender. Separate parameter analysis showed that at least one abnormal electrophysiological parameter was almost always found in patients with retinopathy, macroangiopathy, or incipient nephropathy, but abnormalities were also found to a slightly lesser extent in patients without these complications [10,11]. Finding of the present study as well as the literature support that female gender, advanced age, glycemic control and increased duration of diabetes has a major effect on peripheral nerve function. It suggests that vascular factors may participate in the development of nerve lesion. More than half (126, 57.3%) of the study subjects had hypertension as the major co-morbidity followed by hyperlipidemia (101, 45.9%). However, the present study has not assessed other long term effects of diabetes such as retinopathy and nephropathy [12].
Ample literature supports this finding where patients report pain. Gore M et al., conducted study on pain severity in diabetic peripheral neuropathy reported that great pain levels in DPN is related with higher symptom levels of anxiety and depression, more sleep problems and lower utility rating and physical and mental functioning (p <0.01) [13]. Studies indicate strong association between diabetic neuropathy and foot ulceration [14]. The findings of the present study are in par with the literature where peeling/maceration (30, 13.6%), itching over lower limbs (56, 25.5%), blisters (8, 3.6%) were reported by the subjects [15]. All these are suggestive of the importance of foot care in patients with diabetes mellitus.
The patients with neuropathy are also at risk to develop Charcot neuroarthropathy, a limb-threatening, destructive process that occurs in patients with diabetes mellitus. So, healthcare professionals should be vigilant in recognising the early signs of acute Charcot neuroarthropathy such as pain, warmth, oedema, or pathologic fracture in a neuropathic foot. Prompt treatment if not done, can lead to morbidity and high-level amputation [16]. However, in the present study none of the patients had symptoms of Charcot foot.
The vibration perception threshold demonstrates that majority (152, 69%) of the subjects had a score above 25v which is indicative of severe neuropathy, followed by score between 20-25v which is suggestive of moderate peripheral neuropathy among (31, 14.1%). Normal VPT was recorded only in 12(5.5%). The supporting literature emphasizes that VPT is an active detector to identify diabetic peripheral neuropathy. Rani PK et al., assessed the prevalence and risk factors for severity of DPN in Type II diabetes mellitus among 1401 sample [17]. The study reported a prevalence of 5.9% (95% CI: 4.68-7.15) mild diabetic neuropathy, 7.9% (95% CI: 6.50-9.33) moderate DPN and 5% (95% CI: 3.86-6.14) severe DPN. This findings is in contrast to the findings of the present study where the prevalence of DPN was high among 152 (69%)-VPT value above 25v followed by moderate neuropathy among 31 (14.1%).
The present study gives evidence of a strong association between VPT and sensory symptoms like numbness/tingling, slipping of chapels, pin or needle sensation in the plantar aspect; motor symptoms like muscle cramps, oedema, osteomyelitis; autonomic symptoms like scaly/dry skin, unhealed wound or ulcer; and dermatological symptoms like callus, hyperpigmented plaque/patches, blackish discolouration of toes. Researchers suggest VPT as a definite, specific and sensitive indicator of diabetic peripheral neuropathy. It is considered as a gold standard assessment for neuropathy among patients with diabetes.
Limitation
Generalisation is limited as the study is conducted only in one setting.
Conclusion
From the study findings it can be interpreted that there is a positive correlation between vibration perception threshold values and clinical symptoms of DPN and VPT can be an indicator of severity of diabetic peripheral neuropathy. Every patient with diabetes mellitus is at risk for DPN which needs accurate, proper and early detection. Nurses need to take initiative in reduction and prevention of DPN and make the client aware about the prevention strategy so that amputation can be prevented to a great extent.
DPN: Diabetic peripheral neuropathy
p<0.05, *** significant at <0.001