Clear Cell Sarcoma of Soft Parts (CCSSPs) constitutes approximately 1% of all soft tissue sarcomas and only 300 cases have been described till date. Clear cell sarcoma has overlapping morphology with malignant melanoma and other tumours of soft tissue like epithelioid leiomyosarcoma, epithelioid neurofibrosarcoma, synovial sarcoma, alveolar soft part sarcoma and epithelioid sarcoma. The overlap of morphology and rarity of tumour, brings the diagnosis of CCS under high degree of suspicion. The present series represent cytohistopathological correlation including primary and recurrent CCS with emphasis on diagnostic difficulties and role of ancillary studies.
Case Series
This report concerns six cases of CCS which presented at the Department of Pathology, in a Tertiary Government Hospital, New Delhi, India. Clinical, cytological and histological findings were obtained from patients’ charts and slides. Pertinent data analyses included patient’s age, gender, anatomical location and size of tumour. The operative reports were reviewed in order to classify the operative resections as incisional, marginal, wide, and radical or the level of amputation. However, after the surgical procedure, the follow-up was not done. FNAC smears were stained with Giemsa and Haematoxylin and Eosin (H&E). Histopathological sections were cut and stained with H&E. Masson-Fontana stain was used for demonstration of melanin. Antibodies against Vimentin, S-100 and HMB-45 were used.
The demographic, clinical, radiological, cytologic and histopathologic features of the six patients have been presented in [Table/Fig-1,2 and 3].
The demographic and clinical features of the six patients.
| Case No. | Age | Sex | Tumour location | Clinical presentation with time duration | Swelling | Radiological findings | Pain | Prior treatment |
|---|
| Case 1 | 42 | M | Thigh | Swelling×1 year | Large | Soft tissue swelling with bone involvement | - | Radical excision |
| Case 2 | 23 | M | Thumb | Swelling×2 months | Large | Soft tissue swelling without bone involvement | + | Amputation |
| Case 3 | 45 | M | Wrist | Swelling×2 months | 15×10 cm swelling, ulnar side, involving bone | Soft tissue swelling with bone involvement | + | Excision biopsy |
| Case 4 | 13 | F | Shoulder | Swelling×6 months | 3×5 cm swelling | Soft tissue swelling without bone involvement | - | Excision biopsy |
| Case 5 | 30 | F | Hand | Recurrent Swelling | | Soft tissue swelling without bone involvement | | Wide excision |
| Case 6 | 45 | M | Thigh | Swelling×6 months | 2×2 cm swelling, Gradually progressive×2 months | Soft tissue swelling without bone involvement | + | Excision biopsy |
Pain present: +; Pain absent: -
Cytopathological features.
| Case number | Cellularity | Pleo Morphism | Nucleus | Nucleoli | Cytology | Any other pattern | Intranuc-lear inclusions | Necrosis |
|---|
| Case 1 | Cellular clusters and singly round cells | + | Hyperchromatic | Very few cells | Basophilic, cytoplasmic vacuoles | _ | _ | _ |
| Case 2 | Cellular clusters and singly binucleate cells, round to spindle cells | ++ | Hyperchromatic, eccentric | Few cells | Moderate to abundant basophilic, cytoplasmic vacuoles | Microacinar pattern | _ | _ |
| Case 3 | Cellular clusters and singly MNGs, round to spindle cells | ++ | Hyperchromatic | Few cells show prominent nucleolus | Moderate basophilic | Few acinar structures | _ | + |
| Case 4 | Cellular clusters and singly round cells | + | Hyperchromatic | Few cells | Moderate basophilic and Cytoplasmic vacuoles | _ | _ | _ |
| Case 5 | Cellular clusters and singly round cells | + | Hyperchromatic, eccentric | 2 or 3 less prominent nucleoli | Eosinophilic, granular cytoplasm | Acinar structures + | _ | _ |
| Case 6 | Paucicellular | _ | Hyperchromatic | 2 or 3 less prominent nucleoli | Moderate basophilic | _ | _ | _ |
Present: +; Absent: -
Histopathological features.
| Case no. | Classical pattern | Unusual pattern | Pleomorphism | Epithelioid/MNGs | N/C | Cytoplasm | Nucleolus | Mitoses | Bone | Necrosis | Melanin |
|---|
| Case 1 | + | - | - | +/- | <1 | Vacuoles - | + | 4 | + | + | _ |
| Case 2 | - | Pseudoacinar | + | -/- | 1 | Vacuoles - | + | 3 | _ | _ | _ |
| Case 3 | - | Glandular | ++ | +/- | >1 | Eosinophilic cytoplasm vacuoles | + | 11 | + | + | _ |
| Case 4 | - | Pseudoacinar | +++ | -/+ | >1 | Vacuoles ++ | Focally prominent | 4 | _ | _ | _ |
| Case 5 | - | Microcystic, pseudoacinar | +++ | -/++ | >1 | Granular eosinophilic cytoplasm | Prominent eosinophilic | 6 | _ | _ | _ |
| Case 6 | + | - | ++ | -/- | >1 | Granular eosinophilic cytoplasm | Prominent eosinophilic | 5 | - | - | - |
Present: +; Absent: -
Discussion
The rarity of this tumour is demonstrated by the fact that only few cases have been reported in the literature till date and despite our institute being a tertiary referral center we found only six cases over a 10-year period (2002-2012), wherein the total number of soft tissue sarcomas received were 3000. CCSSP is a rare tumour and only five cases were recorded at the St. Jude’s children Hospital out of total 225 soft tissue sarcomas (over the period of 35 years) in children [1]. Specific recognition of CCSSP in cytological specimens is difficult; due in part to the rarity of this tumour. Definitive diagnosis in the absence of ancillary studies is rarely achieved. Creager AJ et al., and Caraway NP et al., [2,3] had 11 and 9 cases in their cytological reports of CCS. Both the reports showed cytological features comprising of abundant cellularity cells present both in clusters and dispersed singly. These cells were polygonal, rarely fusiform, cells with abundant clear to finely granular cytoplasm, eccentrically placed round nucleus and showed moderate degree of anisonucleosis, single prominent nucleolus and occasionally cells with small multiple nucleoli [2-6].
The features which served as common denominators in these reports were (in contrast to other soft tissue sarcomas with epithelioid morphology simulating CCS), multiple smaller nucleoli [2-6]. These features were observed in our cases too [Table/Fig-4,5]. Intranuclear cytoplasmic inclusions and cytoplasmic pigment have also been described though, not present in our cases [5]. A rare granular cell variant has also been recognised [2]. Tong TR et al., have reported a case of CCS with cells showing marked cellular cohesion and molding, which has not been identified in any of the reported cases of ours [4]. Although, dispersion and decreased cohesion are the characteristic cytological features of these neoplasms [2-6].
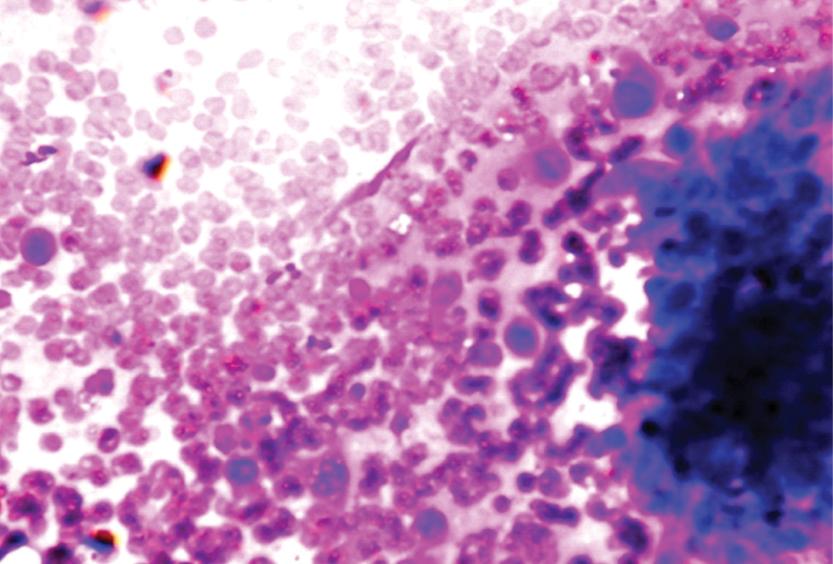
Smear shows cells in clusters and few lying singly. Few cells appear to be epithelioid with prominent eosinophilic nucleoli (arrow). (MGG; 20X) (Case 5).
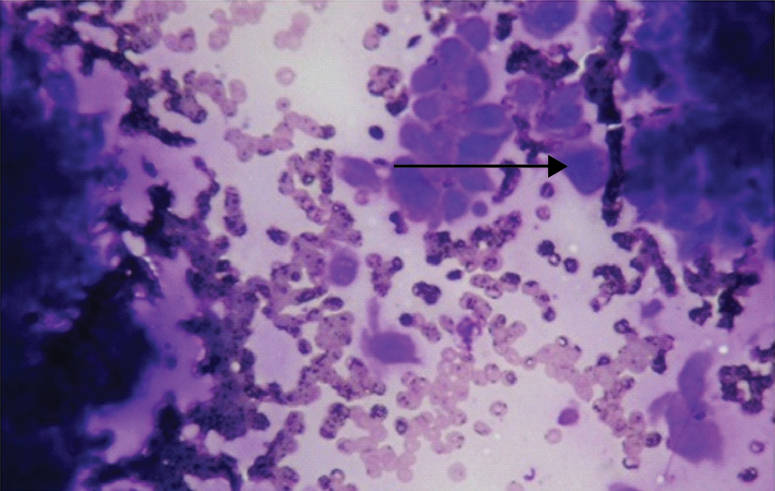
Photomicrograph of CCS showing epithelioid cells with clear to granular cytoplasm and prominent eosinophilic nucleoli (arrow). (MGG; 20X) (Case 3).
A potential cytologic pitfall in the diagnosis of CCS is its ability to mimic metastatic carcinoma by forming cellular aggregates and microacinar structures. They were also identified in one of our case (case 2). Creager AJ et al., also reported similar findings [2].
An incisional biopsy, excisional biopsy, radical excision or amputation may be performed. In present study, three cases (case 3,4,6) had excisional biopsy, one case (case 1) had radical excision to determine healthy margins. One case (case 5) with recurrent swelling underwent surgery elsewhere and we received tissue block for the same. One case (case 2) underwent amputation. The surgical reports were reviewed and the type of operative resection was classified as incisional, marginal, wide or radical on the basis of classification by Simon MA and Enneking WF [7].
Diagnosis of CCSSP on the basis of histomorphology alone is difficult. While the typical case shows the classical pattern of nests of plump cells separated by fibrous stroma [Table/Fig-6,7]. The cells are plump to spindle shaped and have clear cytoplasm [8-10] [Table/Fig-8,9]. Histological variants with a substantial proportion of epithelioid cells with moderate to marked nuclear pleomorphism, predominantly in a diffuse pattern, microcystic pattern (n=1), or alveolar pattern (n=1) were recorded [9]. In addition, one case showed variable number of rhabdoid cells which has been rarely described in CCS, there are very few studies describing similar histological appearance [10-12].
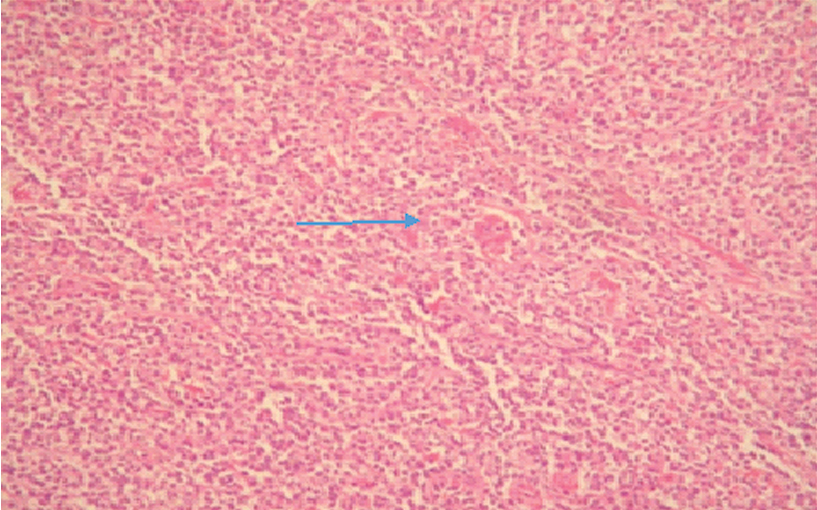
Clear cell sarcoma. Photomicrograph showing uniform cell type (H&E, 4X) (Case 6).
Photomicrograph of CCS showing characteristic round nuclei, prominent nucleoli, and clear cytoplasm (H&E, 40X) (Case 4).
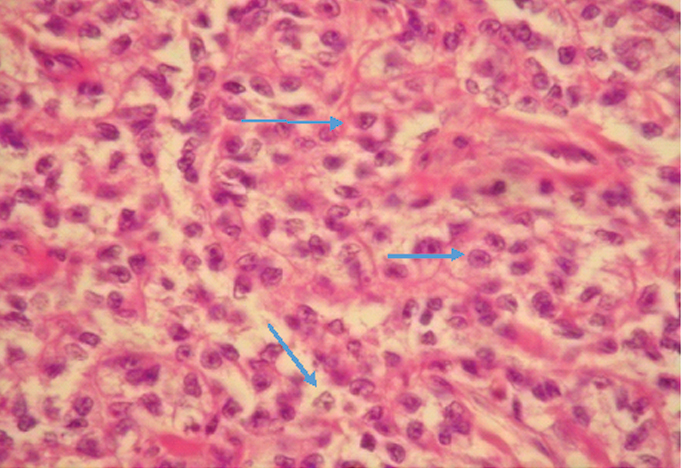
Photomicrograph of CCS showing epithelioid cells with clear to granular cytoplasm and prominent eosinophilic nucleoli. (H&E; 10X) (Case 3).
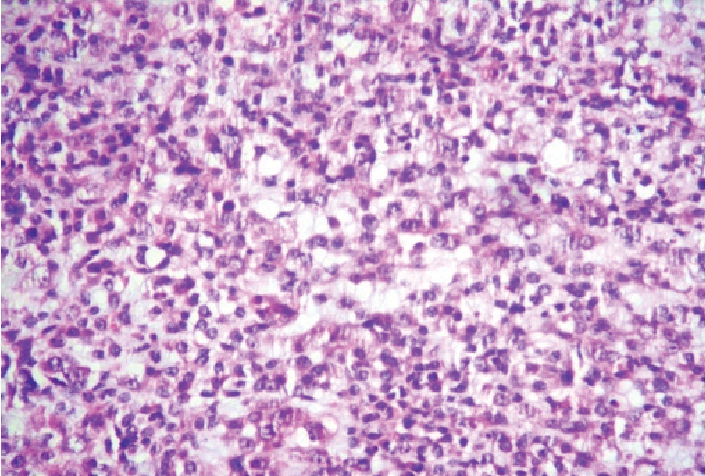
Photomicrograph of CCS showing epithelioid cells with clear to granular cytoplasm. (H&E: 40X) (Case 1).
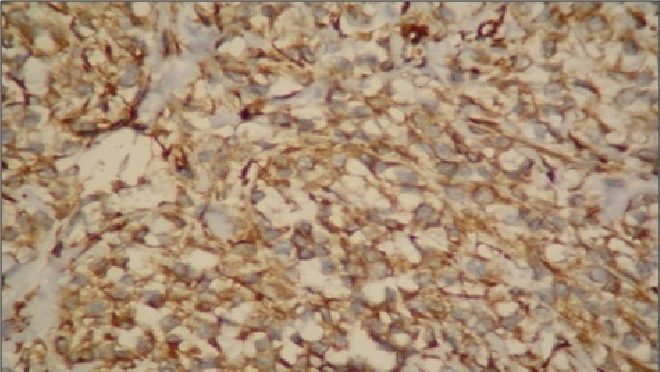
Careful examination is necessary to recognise typical or conventional features of CCS in a given tumour as CCS may show the above unusual histological appearances. It therefore, seems that CCS may display the above unusual histological appearances and scrutiny is necessary to identify the more typical or conventional features of CCS in a given tumour. Melanin was negative in all six cases, even with the use of special stains like Masson-Fontana. While on IHC examination, all patients were positive to HMB-45 antibodies. The reason for the absence of melanin might be the relative paucity of melanin in some clear cell sarcomas. Vimentin was also found positive in all the cases [Table/Fig-10].
Vimentin positivity seen in clear cell sarcoma (40X).
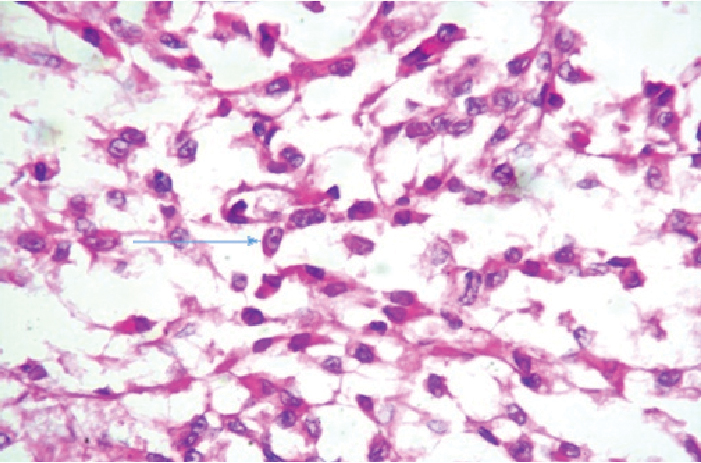
The differential diagnosis in the clinical setting of a young adult with an extremity-based soft tissue mass and regional lymph node metastases includes synovial sarcoma, epithelioid sarcoma, malignant melanoma, fibrosarcoma, rhabdomyosarcoma and paraganglioma.
Synovial sarcoma simulates CCS in its site of origin i.e., both arise in deep soft tissues adjacent to tendons and aponeuroses. Both of them have a tendency to metastasise to regional lymph nodes. On cytology, each subtype demonstrates a different spectrum. The monophasic subtype yields cellular smears showing dispersed, uniform population of ovoid to spindle shaped cells with oval hyperchromatic nuclei, high N/C ratio, small and inconspicuous nucleoli and scant, tapering cytoplasm [13,14]. Biphasic subtypes shows prominent epithelial component. Epithelioid cells are rarely seen on cytological smears, however if present they display abundant, vacuolated cytoplasm [13-15]. IHC profile of synovial sarcoma comprises of cytokeratin, EMA and CD99 expression. Synovial sarcoma is characterised by cytogenetic translocation, t(x;18) (p11;q11) and SYT-SSX fusion gene product which is seen in more than 90% of synovial sarcoma [16].
One of the differential diagnosis is epithelioid sarcoma as it occurs in distal extremities especially the hand [17], because of its predilection for young adults and its ability to metastasise to regional lymph nodes. Its cytomorphologic features show moderately cellular smears and are composed of discohesive and relatively uniform neoplastic cells exhibiting only mild nuclear pleomorphism [14]. Nuclei are round, eccentrically located, and surrounded by slight to moderate amounts of dense cytoplasm [14].
Cells may show intracytoplasmic vacuoles in the background of necrotic debris. IHC of epithelioid sarcoma reveals strong diffuse positivity for cytokeratins and EMA. CD34 is frequently positive in epithelioid sarcoma, rarely seen in carcinomas [18]. It is difficult to distinguish metastatic melanoma from CCS on cytology. However, clinical features play a important role in differentiating the two. Most frequently, CCS is deep seated in extremities of young adults and is associated with tendons and aponeuroses. On the other hand, metastatic melanoma is usually superficial and rarely involves tendons and aponeuroses [2]. There is no difference on immunocytochemistry and t (12;22) has not been found positive in malignant melanoma [19]. Other differential diagnosis include fibrosarcoma, rhabdomyosarcoma, paraganglioma [20]. Rhabdomyosarcoma shows dispersed cells commonly than cell clusters and the cytoplasm is fragile. Stripped nuclei in a blue grey background of smeared cytoplasm are not uncommon. The typical cells resemble myoblasts, being triangular strap shaped or ribbon like with eccentric nuclei, eosinophilic cytoplasm with cytoplasmic vacuolation [21].
Alveolar rhabdomyosarcoma form nests separated by a prominent framework of fibrovascular septa [21]. Majority of tumour cells are poorly differentiated with little cytoplasm and scattered multinucleated tumour giant cells. Immunohistochemistry of rhabdomyosarcoma expresses MSA and desmin. Specific staining with myoD or myogenin should be sought [22]. Paraganglioma-like dermal melanocytic tumour is another important differential diagnosis. It is a tumour which is most commonly seen in extremities of females, presents as a dermal nodule and is composed of clear to amphophilic oval cells. The cells are of low nuclear grade and are arranged in a packet like fashion which is reminiscent of a paraganglioma [23].
Due to the variable morphological features, one must have high degree of skepticism along with IHC to reach the correct diagnosis. Though, ancillary studies are required for accurate diagnosis, these are not available in routine labs, IHC is useful in documenting the melanocytic differentiation. About 80% of cases of CCSSP show S-100 positivity and 75% of cases are HMB 45 positive [24].
In present case series on IHC examination, all cases were positive for HMB-45 antibodies. Recent discoveries include the finding of a characteristic chromosomal translocation, which is unique to CCS, fuelling conflicting interpretations of its classification and histogenesis [18,25]. Obtaining a precise diagnosis of CCSSP is important regarding its treatment. Treatment is primarily surgical. Radical surgery is sometimes postponed in CCSSP until the tumour recurs and this impacts the survival negatively [26]. It is known that patients who develop local recurrence from a CCSSP frequently die of the disease. The prognosis of CCSSP is poor once the tumour metastasises in regional lymph nodes/disseminates hematogeneously. For a favourable outcome, early diagnosis and initial radical surgery are essential [26]. Radiotherapy has a restricted role and is used primarily for residual disease [27]. However, chemotherapy is the mainstay of treatment in the recurrent or metastatic disease of CCSSP1.
Conclusion
Clear cell sarcoma of soft part is a distinct soft tissue sarcoma. Accurate pathologic recognition could aid in the institution of prompt radical surgery and could delay or avoid recurrences.
Pain present: +; Pain absent: -
Present: +; Absent: -
Present: +; Absent: -
[1]. Parasuraman S, Rao BN, Bodnee S, Can A, Pratt CB, Merchant TE, Clear cell sarcoma of soft tissues in children and young adults. The St. Jude Childrens Research Hospital experiencePediate Hematol Oncol 1999 16:539-44.10.1080/08880019927683110599094 [Google Scholar] [CrossRef] [PubMed]
[2]. Creager AJ, Pitman MB, Geisinger KR, Cytologic features of clear cell sarcoma (malignant melanoma) of soft parts. a study of fine needle aspirates and exfoliative specimensAm J Clin Pathol 2002 117:217-24.10.1309/D17Q-2MWA-HVNX-H7RD11863218 [Google Scholar] [CrossRef] [PubMed]
[3]. Caraway NP, Fanning CV, Wojcik CM, Staerkel GA, Benjamin RS, Ordonez NG, Cytology of malignant melanoma of soft parts; fine needle aspirates and exfoliative specimenDiagn Cytopathol 2002 26:174-80. [Google Scholar]
[4]. Tong TR, Chow TC, Chan OW, Lee KC, Yeung SH, Lam A, Clear cell sarcoma diagnosis by fine-needle aspiration: cytologic, histologic, and ultrastructural features, potential pitfalls and literature reviewDiagn Cytopathol 2002 26:174-80.10.1002/dc.1008111892024 [Google Scholar] [CrossRef] [PubMed]
[5]. Kumar N, Das PM, Jan S, Sodhani P, Gupta S, Melanoma of the soft parts: Melanoma of the soft Parts: diagnosis of metastatic and recurrent tumor by aspiration cytologyDiagn Cytopathol 2002 28:295-300.10.1002/dc.1028212768633 [Google Scholar] [CrossRef] [PubMed]
[6]. Almeida MM, Nunes AM, Frable WJ, Malignant melanoma of soft tissue. a report of three cases with diagnosis by fine needle aspiration cytologyActa Cytologica 1994 38:241-46. [Google Scholar]
[7]. Simon MA, Enneking WF, The management of soft tissue sarcomas of the extremitiesJ Bone Joint Surg 1976 58A:31710.2106/00004623-197658030-00005 [Google Scholar] [CrossRef]
[8]. Shabb NS, Boulos F, Tavvil A, Hussein M, Hourani M, Clear cell sarcoma (Malignant Melanoma of soft parts: fine-needle aspiration cytology of a highly pigmented tumorDiagn Cytopathol 2003 28:313-15.10.1002/dc.1028712768636 [Google Scholar] [CrossRef] [PubMed]
[9]. Nguyen GK, Shnitka TK, Jewell LD, Wroblewski JA, Exfoliative cytology of clear cell sarcoma metastases in pleural fluidDiagn Cytopathol 1986 2:144-49.10.1002/dc.28400202093720487 [Google Scholar] [CrossRef] [PubMed]
[10]. Sara AS, Evans HL, Benjamin RS, Malignant melanoma of soft parts (clear cell sarcoma): a study of 17 cases with emphasis on prognostic factorsCancer 1990 65:36710.1002/1097-0142(19900115)65:2<367::AID-CNCR2820650232>3.0.CO;2-X [Google Scholar] [CrossRef]
[11]. Enzinger FM, Clear cell sarcoma of tendons and aponeuroses: an analysis of 21 casesCancer 1965 18:116310.1002/1097-0142(196509)18:9<1163::AID-CNCR2820180916>3.0.CO;2-0 [Google Scholar] [CrossRef]
[12]. Lucas DR, Nascimento AG, Sim FH, Clear cell sarcoma of soft tissue: mayo clinic experience with 35 casesAM J Surg Pathol 1992 16:119710.1097/00000478-199212000-000061463095 [Google Scholar] [CrossRef] [PubMed]
[13]. Kilpatrick SE, Teot LA, Stanley MW, Ward WG, Savage PD, Geisinger KR, Fine-needle aspiration biopsy of synovial sarcoma: a cytomorphologic analysis of primary, recurrent, and metastatic tumorsAm J Clin Pathol 1996 106:769-75.10.1093/ajcp/106.6.7698980353 [Google Scholar] [CrossRef] [PubMed]
[14]. Kilpatrick SE, Geisinger KR, Soft tissue sarcomas: the usefulness and limitations of fine-needle aspiration biopsyAm J Clin Pathol 1998 110:50-68.10.1093/ajcp/110.1.50 [Google Scholar] [CrossRef]
[15]. Akerman M, Willen H, Carlen B, Mandahl N, Mertens F, Fine needle aspiration (FNA) of synovial sarcoma: a comparative histological cytological study of 15 cases, including immunohistochemical, electron microscopic and cytogenetic examination and DNA-ploidy analysisCytopathology 1996 7:187-200.10.1046/j.1365-2303.1996.38782397.x [Google Scholar] [CrossRef]
[16]. Inagaki H, Murase T, Otsuka T, Eimoto T, Detection of SYT-SSX fusion transcript in synovial sarcoma using archival cytologic specimensAm J Clin Pathol 1999 111(4):528-33.10.1093/ajcp/111.4.528 [Google Scholar] [CrossRef]
[17]. Chase DR, Enzinger FM, Epithelioid sarcoma: diagnosis, prognostic indicators, and treatmentAm J Surg Pathol 1985 9:241-63.10.1097/00000478-198504000-00001 [Google Scholar] [CrossRef]
[18]. Arber DA, Kandalaft PL, Mehta P, Battifora H, Vimentin-negative epithelioid sarcoma: the value of an immunohistochemical panel that includes CD34Am J Surg Pathol 1993 17(3):302-07.10.1097/00000478-199303000-000117679559 [Google Scholar] [CrossRef] [PubMed]
[19]. Segal NH, Pavlidis P, Noble WS, Antonescu CR, Viale A, Wesley UV, Classification of clear-cell sarcoma as atype of melanoma by genomic profilingJ Clin Oncol 2003 21:1775-81.10.1200/JCO.2003.10.10812721254 [Google Scholar] [CrossRef] [PubMed]
[20]. Finley JW, Hanypsiak B, McGrath B, Kraybill W, Gibbs JF, Clear cell sarcoma: the Roswell Park experienceJ Surg Oncol 2001 77:16-20.10.1002/jso.105711344475 [Google Scholar] [CrossRef] [PubMed]
[21]. Deenik W, Mooi WJ, Rutgers EJ, Peterse JL, Hart AA, Kroon BB, Clear cell sarcoma (malignant melanoma) of soft parts: a clinicopathological study of 30 casesCancer 1999 86:969-75.10.1002/(SICI)1097-0142(19990915)86:6<969::AID-CNCR11>3.0.CO;2-Z [Google Scholar] [CrossRef]
[22]. Pavlidis NA, Fisher C, Wiltshaw E, Clear cell sarcoma of tendons and aponeurosis; a clinicopathologic studyCancer 1984 54:1412-17.10.1002/1097-0142(19841001)54:7<1412::AID-CNCR2820540730>3.0.CO;2-A [Google Scholar] [CrossRef]
[23]. Fujimura Y, Ohno T, Siddique H, Lee L, Rao N, Reddy ESP, The EWS-ATF-1 gene involved in malignant melanoma of soft parts with t(12;22) chromosome translocation, encodes a consecutive transcriptional activatorOncogene 1996 12:159-67. [Google Scholar]
[24]. Kempson RL, Fletcher CDM, Evans HL, Hendrickon MR, Sibley RK, Tumors of the soft tissuesIn: Atlas of tumor pathology 2001 Washington D.CArmde forces Institute of Pathology:463-67. [Google Scholar]
[25]. David M Parham, Dale A, Ellison (2006) Rhabdomyosarcomas in Adults and Children: An UpdateArchives of Pathology & Laboratory Medicine 2006 130(10):1454-65. [Google Scholar]
[26]. Rangdaeng S, Truong LD, Comparative immunohistochemical staining for desmin and muscle-specific actin. A study of 576 casesAm J Clin Pathol 1991 96(1):32-45.10.1093/ajcp/96.1.32 [Google Scholar] [CrossRef]
[27]. Dim DC, Cooley LD, Miranda RN, Clear cell sarcoma of tendons and aponeurosesA Review Arch Pathol Lab Med 2007 131:152-56. [Google Scholar]