Does the Addition of Melatonin to Quadruple Therapy Increases the Eradication Rate of Helicobacter pylori? A Double-Blind Randomized Clinical Trial
Saeed Abdi1, Mohammad Abbasinazari2, Ghasem Valizadegan3, Mahdieh Kamarei4, Yunes Panahi5, Farhad Sarafzadeh6, Mohammad Amin Pourhoseingholi7
1 Assistant Professor, Gastroenterology and Liver Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2 Professor, Department of Clinical Pharmacy, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3 Assistant Professor, Department of Internal Medicine, Baqiyatallah Research Center for Gastroenterology and Liver Diseases, Tehran, Iran.
4 Student, Department of Clinical Pharmacy, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
5 Professor, Chemical Injuries Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran.
6 Assistant Professor, Department of Infectious Diseases, Afzalipour Hospital, Kerman University of Medical Sciences, Kerman, Iran.
7 Associate Professor, Gastroenterology and Liver Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Mohammad Abbasinazari, Professor, Department of Clinical Pharmacy, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
E-mail: m_abbasi@sbmu.ac.ir
Introduction
The failure in eradication of Helicobacter pylori (H.pylori) using standard treatments is a common concern all over the world. Limited data have shown efficacy of melatonin against H.pylori in vitro. We hypothesise that, melatonin as adjuvant to quadruple therapy may improve H.pylori eradication rate.
Aim
To evaluate additive effects of melatonin combined with a quadruple therapy for the eradication of H.pylori.
Materials and Methods
This was a double-blind, placebo-controlled, randomized clinical trial. The trial comprised a 14-days, quadruple eradication regimen (omeprazole 20 mg twice daily; bismuth subsalicylate 525 mg four times daily; amoxicillin 1000 mg twice daily; and metronidazole 500 mg twice daily) supplemented with melatonin 3 mg/d (MEL group) or a comparable placebo (placebo group) without melatonin. The data were analysed using the SPSS version 19 and p-values less than 0.05 was considered statistically significant.
Results
Eradication rates of intention-to-treat analysis (ITT; n = 118) were 73% in the MEL group and 65% in the placebo group. Eradication rates of per protocol analysis (PP; n = 98) were 80% and 79% in the MEL and placebo groups, respectively. There was no significant difference between the two groups either by ITT or PP analysis (p = 0.74 and p = 0.91, respectively).
Conclusion
According to the result of the trial, MEL 3 mg/d does not have an additive effect on the eradication of H.pylori infection.
Adjunctive therapy, Complementary medicine, Gastrointestinal
Introduction
H.pylori is a highly motile gram-negative bacterium that colonizes the mucus layer of the stomach. It is one of the most common pathogens in humans. It has been recognized as the principle cause of peptic ulcer disease and the main risk factor in the development of gastric cancer [1]. It has been shown that the eradication of H.pylori is associated with an increased rate of peptic ulcer healing and a reduced risk of gastric cancer. Standard triple therapy, which includes a Proton Pump Inhibitor (PPI) and two antibiotics, is recommended as the first-line eradication of H.pylori in clinical guidelines worldwide [2,3]. Increasing rates of antibiotics resistance presents challenges in maintaining optimal eradication rates. Additionally, adverse effect profiles of therapeutic regimens are important and must be addressed to increase adherence rates [4]. Several options for enhancing the eradication rate of H.pylori have been evaluated such as changing combinations or durations of regimens, adding adjuvant medications, or developing new molecules or agents [5]. Melatonin or MEL (N-acetyl-5-methoxytryptamine) is a compound, synthesized mainly by the pineal gland in the human brain. The main action of MEL is the regulation of circadian day-night rhythm and seasonal bio-rhythm by classical chronobiology. There is also evidence that MEL could act as a potent direct antioxidant, a chemo-toxicity reducing agent, and a putative anti-ageing substance [6]. High amounts of MEL are also found in enteroendocrine cells of gastrointestinal mucosa. The amount of MEL in the gut is about 400 times more than the content of MEL in the pineal gland [7]. Benefits of MEL have been reported in the gastrointestinal tract against various irritants, and in healing various lesions like stomatitis, oesophagitis, and peptic ulcers. The major mechanism of anti-ulcer action of MEL includes its cytoprotection, which is a result of its antioxidant and free radical scavenger activity [8]. Klupinska et al., reported that nocturnal secretion of MEL is higher in subjects with asymptomatic infection of H.pylori than in patients infected by H.pylori who had ulcer-like dyspepsia or duodenal ulcers. They have concluded that lower nocturnal secretion of MEL may play a role in the pathogenesis of upper gastrointestinal disorders [9]. The antioxidant potential and immunomodulatory adjuvant activities of MEL have been evaluated in patients infected with H.pylori. In these patients, the levels of mRNA expression of Arylalkylamine-N-Acetyl Transferase (AA-NAT) and Acetyl Serotonin Methyl Transferase (ASMT) were estimated in gastric mucosa and found to have decreased [10]. Chojnacki et al., demonstrated that the decreased expression of MEL-synthesizing enzymes (AA-NAT and ASMT) in patients with symptomatic H.pylori infection returns to a normal level after the eradication of bacteria [11].
In a clinical trial, Celinski et al., concluded that adding MEL or tryptophan to omeprazole treatment significantly accelerates the healing rate of H.pylori-infected chronic gastroduodenal ulcers over that achieved with omeprazole alone [12]. As a result, we can hypothesise that MEL, as an adjuvant to the eradication regimen of H.pylori, may improve the eradication rate of H.pylori. Therefore, we conducted a double-blind randomized clinical trial to assess the efficacy and safety of MEL as an adjuvant to standard quadruple therapy for the eradication of H.pylori infection.
Materials and Methods
This randomized clinical trial was conducted in two referral centers of gastroenterology, affiliated to the Shahid Beheshti University of Medical Sciences and Baqhayatallah University of Medical Sciences, Tehran, Iran from August 2016 to April 2017. The clinical trial was registered in IRCT as IRCT2016082111192N5 and the ethical committee’s approval was obtained from the Shahid Beheshti University of Medical Sciences as per the provisions of Helsinki [13]. Also, all the subjects signed consent form in Persian language after the study procedures were thoroughly explained. Patients with any kind of upper gastrointestinal disorders who were H.pylori positive and eligible for eradication were selected for the study.
Diagnosis of H.pylori was determined by a rapid urease test via an endoscopy {Pronto Dry (GASTREX, Warsaw, Poland)}. Exclusion criteria included refusal to enter the study; being under 18 years of age; presence of severe comorbidities; history of gastric surgery; prior H.pylori eradication therapy; intake of any antibiotics; PPIs; histamine (H2) receptor blockers; bismuth salts taken within the last month; being allergic toward any of the antibiotics used in the study; active upper gastrointestinal bleeding; and pregnant or breastfeeding women. Each participant completed a questionnaire which included their demographic data. Eligible patients (put randomly in a 1:1 proportion, using a sealed envelope containing a therapeutic option derived from a computer-generated randomization table) were to receive one of the following treatments – a 14-day quadruple therapy regimen consisting of omeprazole 20 mg twice daily, bismuth subsalicylate 525 mg 4 times daily, amoxicillin 1000 mg twice daily, and metronidazole 500 mg twice daily supplemented with MEL 3 mg (MEL group) or a placebo, which was identical (14 days quadruple therapy regimen) to active MEL (placebo group) in order to assess concealed allocation. This is the most frequent quadruple therapy administered by Iranian gastroenterologists. Both the active treatment and the placebo were taken orally on a daily basis for 14 days.
Eradication of H.pylori was evaluated, 28 days after the treatment course was completed, by a stool antigen test. A positive result on the stool antigen test, 28 days after completion of therapy, identifies patients in whom the eradication of H.pylori has been unsuccessful [14].
Patients’ adherence and ADRs were determined in studied patients. Therapy adherence was considered in patients who reported that they took more than 80% of their tablets. A pharmacist was the only person with knowledge of the assignment code and she had no contact with the study staff. She also checked the adherence and ADRs of all patients over the phone upon completion of the 14-days therapy.
The sample size of each group was calculated according to our data, which showed that the eradication rate through quadruple therapy in Iran was 70% [15]. Using 80% power (β =80%) at a 5% significance level (α =0.05), a 30% difference was detected in eradication rates between quadruple therapy with MEL or with placebo. At least 84 patients were necessary for this trial (42 in each group). The data were analysed using the SPSS version 19 (IBM SPSS, Chicago, IL, USA). The Chi-square test for categorical variables and student’s t-test or Mann-Whitney test for continuous variables were used for testing differences between the two groups. A significance level of <0.05 was used in this test.
Results
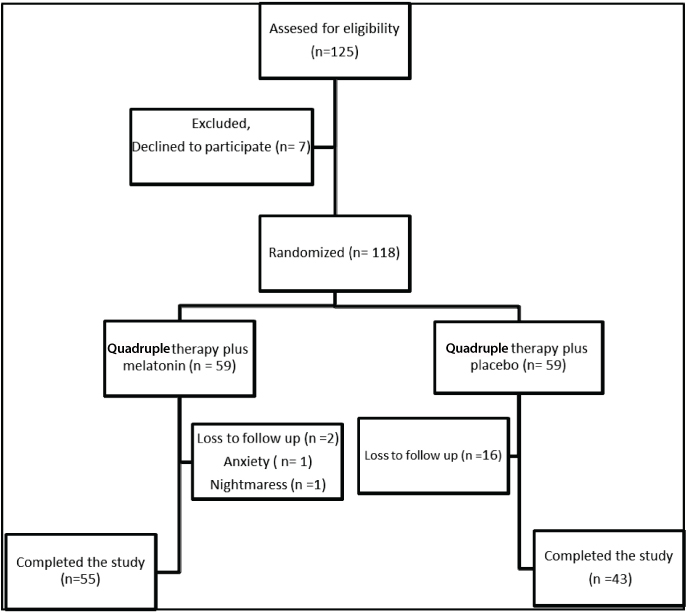
Among the 125 eligible patients initially enrolled in this trial, seven were excluded due to their refusal to participate; as a result, a total of 118 patients participated in this study. The mean age of the participants was 46.6±15.1 and 56.1% were females. The flowchart of participants throughout the study is shown in [Table/Fig-1]. [Table/Fig-2] provides clinical variables for the MEL and placebo groups. There was no significant difference in mean age between the two groups (p = 0.62). Also, the two groups had similar gender distribution; the MEL group had 56.8% males and the placebo group had 43.1% males (p = 0.86). Also, no significant differences were noted in smoking habits and diagnosis between the MEL and placebo groups (p =0.85 and p= 0.52, respectively).
Demographic data of studied patients.
| Total (n=118) | MEL group (n=59) | Placebo group (n=59) | p |
|---|
| Mean Age (±SD) | 46.6±15.1 | 47.2±16.3 | 45.7±13.5 | 0.62 |
| Sex (Female/ Male) | 50/68 | 24/35 | 26/33 | 0.86 |
| Smokers (No/Yes) | 103/15 | 54/5 | 49/10 | 0.85 |
| Diagnosis (NUD/ PUD) | 96/22 | 50/9 | 46/13 | 0.52 |
NUD: Non Ulcer Dyspepsia, PUD: Peptic Ulcer Disease
A total number of 118 patients undergoing H.pylori eradication regimen were included in the Intention To Treat (ITT) analysis and 98 patients were included in the Per Protocol (PP) analysis. Eradication rates of both groups are shown in [Table/Fig-3]. Although eradication rates in the MEL group were a little higher than in the placebo group (73% versus 65% in ITT; 80% versus 79% in PP), analysis showed that there is no significant difference between the ITT and PP groups (p= 0.74 and p = 0.91, respectively). Two patients in the MEL group dropped out during the study because of nightmares and anxiety. All the 98 patients (in the PP analysis) had an adherence rate of more than 80%. Except two cases who experience anxiety and nightmares during the study in MEL group, there were not any ADRs in studied patients.
Eradication rate of patients via ITT and PP analysis.
| Variable | Quadruple therapy plus MEL | Quadruple therapy plus placebo | p |
|---|
| Patients (n) | Eradication rate % | Patients (n) | Eradication rate % |
|---|
| ITT | 59 | 73% | 59 | 65% | 0.74 |
| PP | 55 | 80% | 43 | 79% | 0.91 |
ITT: Intention To Treat, PP: Per Protocol
Discussion
In recent years, the eradication rate for H.pylori has decreased; recent studies have shown that the eradication rate has dropped below 85% in the US and in Europe [16]. Failure in the eradication of H.pylori in Iran has been reported in a systematic review by Khademi et al., [17]. They have reported that rates of H.pylori resistance to various antibiotics including metronidazole, clarithromycin, amoxicillin, tetracycline, ciprofloxacin, levofloxacin, and furazolidone were 61.6%, 22.4%, 16.0%, 12.2%, 21.0%, 5.3%, and 21.6%, respectively [17]. Therefore, optimizing H.pylori eradication therapy remains an ongoing challenge. One strategy is the addition of an adjuvant to the eradication regimen of H.pylori in order to increase the rate of eradication. Nseir et al., have evaluated the effect of adding simvastatin to the triple regimen in order to determine whether this adjuvant agent will improve the eradication rate. Although the mechanism of anti-H.pylori effect of simvastatin has not yet been understood, they have reported that the addition of simvastatin to standard triple therapy significantly improves the eradication rate of H.pylori eradication [18]. The beneficial gastroprotective effect of MEL has been reported on experimental animals [19,20]. Limited data are available regarding the effect of MEL on the eradication of H.pylori in humans. This trial has been conducted to determine the effect of MEL on the eradication rate of H.pylori. Studied patients were similar in terms of mean age, sex, smoking habits, and diagnosis.
Osadchuk et al., have evaluated the effect of adding MEL 3 mg/d to a triple therapy (amoxicillin, clarithromycin, and omeprazole) in 100 patients diagnosed with duodenal ulcers who were H.pylori positive. The patients continued MEL consumption for two months. It was shown that the introduction of MEL in the triple therapy increases the efficacy of H.pylori elimination and accelerates duodenal ulcer cicatrisation [21]. However, in our study, patients continued MEL only during quadruple therapy (two weeks). Perhaps a shorter duration of MEL usage does not lead to any benefit in the MEL group in our study as compared to the study by Osadchuk et al. Like Osadchuk et al., we also administered the same dose (3 mg/d) in addition to a quadruple therapy in the present trial. It is possible that this dose is not strong enough for a positive response. For example, Celinski et al., have compared the effects of administration of 10 mg/d of MEL plus omeprazole 40 mg/d versus omeprazole 40 mg/d in limited patients with peptic ulcer disease who were H.pylori positive. They reported a better acceleration of ulcer healing in the combinational group. Unfortunately, they have not reported the eradication rate of H.pylori in their study [12]. A low oral bioavailability (between 9% and 33%) has been reported for MEL in pharmacokinetic studies. This may be a result of poor absorption from gastrointestinal tract, an extensive first pass metabolism in the liver, or from a combination of both [22].
Also, there are some factors that affect the MEL level in a human body. For example, Ursing et al., have concluded that smoking may change the MEL level [23], although patients had similar smoking habits in our study. Further studies need to evaluate wheather an increase in MEL usage can increase the eradication rate of H.pylori or not.
Conclusion
We have concluded that MEL in a dose of 3 mg/d added during a course of quadruple therapy (including bismuth, amoxicillin, metronidazole, and omeprazole) could not increase the eradication rate.
NUD: Non Ulcer Dyspepsia, PUD: Peptic Ulcer Disease
ITT: Intention To Treat, PP: Per Protocol
[1]. Moss SF, The clinical evidence linking Helicobacter pylori to gastric cancerCell Mol Gastroenterol Hepatol 2017 3(2):183-91.10.1016/j.jcmgh.2016.12.00128275685 [Google Scholar] [CrossRef] [PubMed]
[2]. Xin Y, Manson J, Govan L, Harbour R, Bennison J, Watson E, Pharmacological regimens for eradication of Helicobacter pylori: An overview of systematic reviews and network meta-analysisBMC Gastroenterol 2016 16(1):80-96.10.1186/s12876-016-0491-727460211 [Google Scholar] [CrossRef] [PubMed]
[3]. Khandouzi N, Shidfar F, Agah Sh, Hosseini A, Dehnad A, Comparison of the effects of Eicosapentaenoic acid and Docosahexaenoic acid on the eradication of Helicobacter pylori infection, serum inflammatory factors and total antioxidant capacityIran J Pharmaceut Res 2015 14(1):149-57. [Google Scholar]
[4]. Abbasinazari M, Sahraee Z, Mirahmadi M, The patients’ adherence and adverse drug reactions (ADRs) which are caused by Helicobacter pylori eradication regimensJ Clin Diagn Res 2013 7(3):462-66.10.7860/JCDR/2013/4673.2799PMC3616557 [Google Scholar] [CrossRef] [PubMed]
[5]. Bang CS, Baik GH, Attempts to enhance the eradication rate of Helicobacter pylori infectionWorld J Gastroenterol 2014 20(18):5252-62.10.3748/wjg.v20.i18.525210.3748/wjg.v20.i18.5252PMC4017040 [Google Scholar] [CrossRef] [PubMed] [PubMed]
[6]. Carpentieri A, Díaz De Barboza G, Areco V, Peralta López M, Tolosa De Talamoni N, New perspectives in MEL usesPharmacol Res 2012 65(4):437-44.10.1016/j.phrs.2012.01.00322311380 [Google Scholar] [CrossRef] [PubMed]
[7]. Bubenik GA, Thirty four years since the discovery of gastrointestinal MELJ Physiol Pharmacol 2008 59(2):33-51. [Google Scholar]
[8]. Konturek SJ, Konturek PC, Brzozowski T, Bubenik GA, Role of MEL in upper gastrointestinal tractJ Physiol Pharmacol 2007 58(SUPPL. 6):23-52. [Google Scholar]
[9]. Klupinska G, Chojnacki C, Harasiuk A, Stȩpień A, Wichan P, Stec-Michalska K, Nocturnal secretion of MEL in subjects with asymptomatic and symptomatic Helicobacter pylori infectionPol Merkuriusz Lek 2006 21(123):239-42. [Google Scholar]
[10]. Vielma JR, Bonilla E, Chacín-Bonilla L, Mora M, Medina-Leendertz S, Bravo Y, Effects of melatonin on oxidative stress, and resistance to bacterial, parasitic, and viral infections: A reviewActa Trop 2014 137:31-38.10.1016/j.actatropica.2014.04.02124811367 [Google Scholar] [CrossRef] [PubMed]
[11]. Chojnacki C, Poplawski T, Blasiak J, Chojnacki J, Reiter RJ, Klupinska G, Expression of melatonin synthesizing enzymes in Helicobacter pylori infected gastric mucosaBiomed Res Int 2013 2013:84503210.1155/2013/84503223936850 [Google Scholar] [CrossRef] [PubMed]
[12]. Celinski K, Konturek PC, Konturek SJ, Slomka M, Cichoz-Lach H, Brzozowski T, Effects of MEL and tryptophan on healing of gastric and duodenal ulcers with Helicobacter pylori infection in humansJ Physiol Pharmacol 2011 62(5):521-26.10.1016/S0016-5085(12)61877-0 [Google Scholar] [CrossRef]
[13]. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjectsJAMA 2013 310:2191-94.10.1001/jama.2013.28105324141714 [Google Scholar] [CrossRef] [PubMed]
[14]. Vaira D, Vakil N, Menegatti M, Van’t Hoff B, Ricci C, Gatta L, The stool antigen test for detection of Helicobacter pylori after eradication therapyAnn Intern Med 2002 136(4):280-87.10.7326/0003-4819-136-4-200202190-0000711848725 [Google Scholar] [CrossRef] [PubMed]
[15]. Amini M, Khedmat H, Yari F, Eradication rate of Helicobacter pylori in dyspeptic patientsMed Sci Monit 2005 11(4):CR193-95. [Google Scholar]
[16]. Raymond J, Lamarque D, Kalach N, Chaussade S, Burucoa C, High level of antimicrobial resistance in French Helicobacter pylori isolatesHelicobacter 2010 15(1):21-27.10.1111/j.1523-5378.2009.00737.x20302586 [Google Scholar] [CrossRef] [PubMed]
[17]. Khademi F, Poursina F, Hosseini E, Akbari M, Safaei HG, Helicobacter pylori in Iran: A systematic review on the antibiotic resistanceIran J Basic Med Sci 2015 18(1):2-7. [Google Scholar]
[18]. Nseir W, Diab H, Mahamid M, Abu-Elheja O, Samara M, Abid A, Randomised clinical trial: Simvastatin as adjuvant therapy improves significantly the Helicobacter pylori eradication rate - A placebo-controlled studyAliment Pharmacol Ther 2012 36(3):231-38.10.1111/j.1365-2036.2012.05161.x [Google Scholar] [CrossRef]
[19]. Cabeza J, Alarcón-De-La-Lastra C, Jiménez D, Martín M-, Motilva V, Melatonin modulates the effects of gastric injury in rats: Role of prostaglandins and nitric oxideNeurosignals 2003 12(2):71-77.10.1159/00007181612876401 [Google Scholar] [CrossRef] [PubMed]
[20]. Sener-Muratoglu G, Paskaloğlu K, Arbak S, Hürdag C, Ayanoglu-Dülger G, Protective effect of famotidine, omeprazole, and melatonin against acetylsalicylic acid-induced gastric damage in ratsDig Dis Sci 2001 46(2):318-30.10.1023/A:100565281592111281181 [Google Scholar] [CrossRef] [PubMed]
[21]. Osadchuk MA, Sibriaev AA, Kireeva NV, Kvetnoi IM, The influence of melatonin included in the combined treatment of Anti-Helicobacterial therapy on immunohistochemical characteristics of gastric epitheliocytes from patients with duodenal ulcerKlin Med (Mosk) 2012 90(12):48-52. [Google Scholar]
[22]. Harpsøe NG, Andersen LPH, Gögenur I, Rosenberg J, Clinical pharmacokinetics of melatonin: A systematic reviewEur J ClinPharmacol 2015 71(8):901-09.10.1007/s00228-015-1873-426008214 [Google Scholar] [CrossRef] [PubMed]
[23]. Ursing C, Von Bahr C, Brismar K, Röjdmark S, Influence of cigarette smoking on melatonin levels in manEur J Clin Pharmacol 2005 61(3):197-201.10.1007/s00228-005-0908-715824912 [Google Scholar] [CrossRef] [PubMed]