Clinico Pathological Analysis of Uterine Serous Carcinoma in a Cancer Centre from Northern Kerala-A Retrospective Study
Indusarath1, Aswathi Krishnan2, Sangeetha K Nayanar3, Sampada Dessai4, Maya Padmanabhan5
1 Assistant Professor, Department of Pathology, Kannur Medical College, Kannur, India.
2 Assistant Professor, Department of Onco-Pathology, Malabar Cancer Centre, Thalassery, Kerala, India.
3 Professor, Department of Onco-Pathology, Malabar Cancer Centre, Thalassery, Kerala, India.
4 Associate Professor, Department of Surgical Oncology, Malabar Cancer Centre, Thalassery, Kerala, India.
5 Lecturer in Biostatistics, Malabar Cancer Centre, Thalassery, Kerala, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Indusarath, Assistant Professor, Department of Pathology, Kannur Medical College, Kannur-670612, Thalassery, Kerala, India.
E-mail: indusarath@gmail.com
Introduction
Uterine Serous Carcinoma (USC) accounts for 10% of malignancies of uterine corpus. They are characterised by high propensity for local spread, extra uterine metastasis, recurrence and are having poor prognosis. The disease can be highly aggressive even if it is confined to uterus at time of surgery.
Aim
To assess the clinico-pathologic features of uterine serous carcinoma in patients of north Kerala and to analyse its predictors of outcome.
Materials and Methods
Data of cases diagnosed with uterine serous carcinoma for five years from 2012 January to 2016 December were retrieved from medical library. Overall survival and progression free survival were assessed.
Results
It is observed that all patients were post menopausal women with the age of 59.5+8.535 with predominant clinical presentation of vaginal bleeding. As per the FIGO staging, 13 patients were in Stage I, 11 cases were in Stage III, 4 cases were in IV and no cases reported in Stage II. Out of 28, 9 cases (32.1%) were died and 12 patients (42.8%) had recurrence during the follow-up period. 19 cases (67.9 %) had the five year over all survival. For stage I patients the overall survival was 76.9%, Stage III had 72.7%, and Stage IV had only 25%. 3 out of the 13 cases in Stage I, 6 out of 11 cases in Stage III and 3 out of 4 cases in Stage IV showed recurrence irrespective of the adjuvant treatment.
Conclusion
Uterine serous carcinoma has distinct clinico-pathologic features compared to the more common endometrioid carcinoma. It carries poor prognosis. Complete surgical staging with close follow-up is needed even in cases confined to uterus.
Endometrial serous carcinoma, Prognosis, Surgical staging, Type II endometrial carcinoma
Introduction
Uterine Serous Carcinoma is an uncommon malignancy of uterine corpus with aggressive behaviour [1]. It is a prototypical Type II endometrial tumour [2]. Even though the prevalence is 10% of all uterine malignancies, it accounts for 40% of endometrial cancer related mortality and morbidity [3]. FIGO System is used for staging of all Endometrial malignancies [4]. Hendrickson M et al., in 1982 reported the first large series of USC which showed 13 relapses in 26 women with pathological Stage I disease [5]. Subsequent studies have confirmed survival rates of 35-50% for Stage I-II and 0-15% for Stage III-IV [6-9]. Recurrence rates for USC are high (50-80%) even when disease is confined to the uterus [10,11]. We conducted a retrospective study on the disease in patients treated at a single cancer institution to determine predictors of outcome.
Materials and Methods
A retrospective study was conducted. A total of 117 patients were diagnosed as having carcinoma endometrium during 2012 to 2016 in Malabar Cancer Centre, Thalassery, Kerala, India. It included 83 cases of endometrioid adenocarcinoma, 31 cases of pure serous carcinoma and 3 cases of clear cell carcinoma. Out of this, 31 cases of serous carcinoma or mixed endometrial carcinoma with at least 5% serous component were selected for the study. Details of these 31 cases were retrieved from the archival files of Malabar Cancer Centre, Thalassery, after obtaining the approval of institutional review board. Out of the 31 patients, 3 patients who had not undergone surgical staging due to poor general condition or very advanced disease were excluded from the study. Remaining 28 cases were included in the study. Demographic information including age at diagnosis, parity, age at menarche and menopause, other co-morbidities, significant past and family history, treatment, disease progression and survival status were obtained from the medical records. Surgical pathology reports were reviewed to obtain histopathologic data. The aim and objective of the study was to assess the clinico-pathologic features of uterine serous carcinoma. The primary end points were to find out the Overall Survival (OS) and Progression-Free Survival (PFS).
Statistical Analysis
The statistical analysis was done by using SPSS version 17.0 software. The data were expressed by using number, percentage, Mean±SD. The OS and PFS were calculated from the date of the surgical diagnosis. OS and PFS were estimated on the basis of Kaplan-Meier curves.
Results
The study cohort consisted of 28 patients. Mean age at presentation was 59.5 years (range, 44-76 years; 25th/75th percentile, 52.25-66 years). All the patients were post menopausal. Most common symptom in any stage disease was abnormal vaginal bleeding (89.3%). Other clinical presentations were abdominal pain and uterine prolapse. None of the patients were having any past history of malignancy. Five patients had family history of malignancy in first degree relative out of which two had breast cancer (7%). Histologically uterine serous carcinoma is characterised predominantly by papillae, also glandular architecture and solid sheets seen. Each fibrovascular papilla is lined by epithelial cells with large atypical nuclei pleomorphic cells [Table/Fig-1a-c]. In 13 cases, the IHC for p16 was done; all of them showed diffused strong nuclear and cytoplasmic positivity [Table/Fig-1d]. Thirteen of the 28 patients in the study (46.4%) had Stage I disease {IA, 6 (21.4%); IB, 7 (25%)}, none of the patients had Stage II disease, 11 patients (39.3%) had Stage III disease (IIIA, 1 (3.8%); IIIB, 2 (7.1%); and IIIC, 8 (28.6%)), and 4 patients (14.3%) had stage IVB disease. Three of them had peritoneal deposits. The pathological characteristics in each stage are depicted in [Table/Fig-2].
a) Endometrial tumour invading myometrium, 40x, H&E; b) Tumour cells are arranged in glands, papillae and in cords, 100x, H&E; c) Individual tumour cells are having pleomorphic vesicular nucleus with prominent nucleoli, 400x, H&E; d) Tumour cells are positive for p16, which is unrelated to HPV infection, 400x, IHC.

Pathological characteristics in each stage. Stage I [13] includes A [6], B [7]; Stage III [11] includes A[1], B[2],C1[7],C2[1]; Stage IV includes A [0], B[4]
| Characteristics | Number of patients (%) |
|---|
| All patients [28] | Stage (Total) |
|---|
| I [13] | III [11] | IV [4] |
|---|
| Depth of myometrial invasion | >50% | 20 (71.4) | 7 (53.8) | 10 (90.9) | 3 (75) |
| <50% | 8 (28.6) | 6 (46.1) | 1 (9.1) | 1 (25) |
| Lymphovascular invasion | 9 (32.1) | 1 (7.7) | 6 (54.5) | 2 (50) |
| Histology | Pure | 20 (71.4) | 10 (76.9) | 7 (63.6) | 3 (75) |
| Mixed | 8 (28.6) | 3 (23.1) | 4 (36.4) | 1 (25) |
| Necrosis | 16 (57.1) | 7 (53.8) | 7 (63.6) | 2 (50) |
| Lymph node involvement | 12 (42.9) | 0 (0) | 8 (72.7) | 4 (100) |
| Peritoneal cytology | 4 (14.3) | 0 (0) | 1 (9.1) | 3 (75) |
In 13 cases, the IHC for p16 was done; all of them showed diffused strong nuclear and cytoplasmic positivity [Table/Fig-1d]. The mean follow-up duration was 24 months (ranging from 9-55 months). Out of 28 patients, 9 patients died during the follow-up period. The mean overall survival in all stage was 26.6 months. The overall survival in each stage was plotted with Kaplan-Meier curve [Table/Fig-3].
Kaplan-Meier survival plot to assess the overall survival. The OS of Stage I is very low compared to Stage IV (p=0.036). It can be due to the skewing by a single case of Stage IV with OS of 44 months.
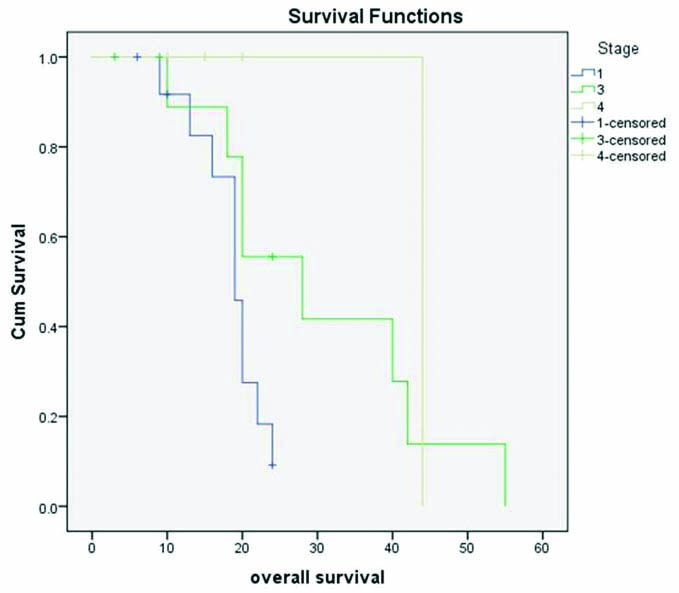
Twelve (42.8%) patients had progression of the disease, mainly as para-aortic lymph node metastasis (5/12) [Table/Fig-4]. Out of the 12 cases that had a progression/recurrence: 3 were in Stage I (3/13; 23%); 6 in Stage III (54.5%); and 3 in Stage IV (75%). The mean progression free survival was 19.9 months. Stage I, III and IV had 18.3 months, 20.7 months and 24 months respectively. However, the difference in progression free survival in each stage showed no significance on analysis Log Rank test (p=0.457).
Table showing the sites of recurrence.
| Sites of recurrence | Count |
|---|
| Para-aortic LN | 5 |
| SCLN | 2 |
| Liver | 1 |
| Peritoneum | 1 |
| Rectum | 1 |
| Mesentery | 1 |
| Pleural fluid | 1 |
Discussion
Uterine serous carcinomas are rare and the patients have poor prognosis. Histologically, these tumours more closely resemble serous carcinoma of the ovary and have distinctly different clinical behaviour compared to the more common endometrioid adenocarcinoma of the endometrium [12]. USC have been characterised by high propensity for extra uterine metastasis, intraperitoneal spread, high risk of recurrence and poor prognosis [13]. Studies have shown that racial origin is important in incidence and survival rates of USC [14]. Considering the rarity of the tumour and inadequate amount of studies conducted in our region (Kerala), we believe the present study will contribute to better understanding of the behaviour of the tumour in the region.
The prevalence of uterine serous carcinoma is 25.2% in our study involving patients from north Kerala which is high compared to studies done in other places [1,3,15]. The mean age of the patients at diagnosis was 59.5 years which is low, compared to other studies [16]. However, this value is comparable with the results observed by Song T et al., in a study conducted in Korea [17]. The extra uterine disease spread at the time of diagnosis was 53.5%. Abnormal vaginal bleeding in the post menopausal woman was the most common clinical feature similar to endometrioid adenocarcinoma. Woman with past history of breast cancer has increased risk of developing endometrial carcinoma. It may be due to an underlying genetic predisposition rather than oestrogen sensitivity [18]. In our study none of the patients had breast carcinoma but 2 of them had first degree relatives with the same. Lymphovascular emboli and myometrial invasion are the significant factors which affect the prognosis. Out of the 8 cases with myometrial invasion of less than 50%, one case had adnexal involvement (T3b) and 2 showed pelvic lymph node metastasis (N1). This signifies the importance of a complete surgical staging in all cases of endometrial serous carcinoma. Our study showed that mixed carcinomas with serous component will behave similar to the pure serous carcinoma. Studies done by Slomovitz BM et al., Sherman ME et al., had the same impression [16,19].
The 5 year overall survival for all cases was 32.1%. For Stage I patients, it was 76.9% (stage IA 83.3%, stage IB 71.4%). Stage III had 72.7%, and Stage IV had only 25% of 5 year OS. 3 out of the 13 cases in Stage I showed recurrence as para-aortic LN metastasis and peritoneal deposits even after adequate para-aortic sampling done during surgical staging.
In this single institution study, we are able to observe a poor survival rate and a lower average diagnosis age as compared to studies done elsewhere [16,20]. At the same time, there are some similarities between our study and study conducted in another Asian country especially in the diagnosis age [17]. Sherman ME et al., in their study has shown that factors such as racial background affect survival rates considerably in case of malignant tumours of uterine corpus [21]. Our observations point to the need for more detailed analysis, possibly involving a larger sample group, about the significance of ethnicity and socio-economic conditions in the incidence and survival rates of USC.
Conclusion
Uterine serous carcinomas have a distinct pathological and clinical characteristic from endometrioid carcinoma. So it should not be evaluated in the same manner as the latter. Even the patients with tumour confined to the uterus will have poor prognosis. Complete surgical staging is mandatory for all cases. The prevalence of this tumour is high in north Kerala compared to studies done in other places and the age at diagnosis is also lower. However, the results need to be validated in a larger sample with prospective randomised controlled clinical trial.
[1]. Creasman WT, Odicino F, Maisonneuve P, Beller U, Benedet JL, Heintz AP, Carcinoma of the corpus uteriJ Epidemiol Biostat 2001 6(1):47-86. [Google Scholar]
[2]. Carcangiu M, Kurman R, Carcangiu M, Herrington C, WHO Classification of Tumours of Female Reproductive Organs 2014 LyonInternational Agency for Research on Cancer [Google Scholar]
[3]. Fader AN, Boruta D, Olawaiye AB, Gehrig PA, Uterine papillary serous carcinoma: epidemiology, pathogenesis and managementCurr Opin Obstet Gynecol 2010 22(1):21-29.10.1097/GCO.0b013e328334d8a319952744 [Google Scholar] [CrossRef] [PubMed]
[4]. Creasman W, Revised FIGO staging for carcinoma of the endometriumInt J Gynaecol Obstet 2009 May 105(2):10910.1016/j.ijgo.2009.02.01019345353 [Google Scholar] [CrossRef] [PubMed]
[5]. Hendrickson M, Ross J, Eifel PJ, Cox RS, Martinez A, Kempson R, Adenocarcinoma of the endometrium: analysis of 256 cases with carcinoma limited to the uterine corpus. Pathology review and analysis of prognostic variablesGynecol Oncol 1982 13(3):373-92.10.1016/0090-8258(82)90076-2 [Google Scholar] [CrossRef]
[6]. Abeler VM, Kjørstad KE, Serous papillary carcinoma of the endometrium: a histopathological study of 22 casesGynecol Oncol 1990 39(3):266-71.10.1016/0090-8258(90)90250-O [Google Scholar] [CrossRef]
[7]. Sutton GP, Brill L, Michael H, Stehman FB, Ehrlich CE, Malignant papillary lesions of the endometriumGynecol Oncol 1987 27(3):294-304.10.1016/0090-8258(87)90249-6 [Google Scholar] [CrossRef]
[8]. Ramirez-Gonzalez CE, Adamsons K, Mangual-Vazquez TY, Wallach RC, Papillary adenocarcinoma in the endometriumObstet Gynecol 1987 70(2):212-15. [Google Scholar]
[9]. O’Hanlan KA, Levine PA, Harbatkin D, Feiner C, Goldberg GL, Jones JG, Virulence of papillary endometrial carcinomaGynecol Oncol 1990 37(1):112-19.10.1016/0090-8258(90)90318-F [Google Scholar] [CrossRef]
[10]. Christman JE, Kapp DS, Hendrickson MR, Howes AE, Ballon SC, Therapeutic approaches to uterine papillary serous carcinoma: a preliminary reportGynecol Oncol 1987 26(2):228-35.10.1016/0090-8258(87)90278-2 [Google Scholar] [CrossRef]
[11]. Jeffrey JF, Krepart GV, Lotocki RJ, Papillary serous adenocarcinoma of the endometriumObstet Gynecol 1986 67(5):670-74.10.1097/00006250-198605000-000133960439 [Google Scholar] [CrossRef] [PubMed]
[12]. Sherman ME, Bitterman P, Rosenshein NB, Delgado G, Kurman RJ, Uterine serous carcinoma. A morphologically diverse neoplasm with unifying clinicopathologic featuresAm J Surg Pathol 1992 16(6):600-10.10.1097/00000478-199206000-000081599038 [Google Scholar] [CrossRef] [PubMed]
[13]. Carcangiu ML, Chambers JT, Uterine papillary serous carcinoma: a study on 108 cases with emphasis on the prognostic significance of associated endometrioid carcinoma, absence of invasion, and concomitant ovarian carcinomaGynecol Oncol 1992 47(3):298-305.10.1016/0090-8258(92)90130-B [Google Scholar] [CrossRef]
[14]. Long B, Liu FW, Bristow RE, Disparities in uterine cancer epidemiology, treatment, and survival among African Americans in the United StatesGynecol Oncol 2013 130(3):652-59.10.1016/j.ygyno.2013.05.02023707671 [Google Scholar] [CrossRef] [PubMed]
[15]. Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancersBr J Cancer 2006 94(5):642-46.10.1038/sj.bjc.660301216495918 [Google Scholar] [CrossRef] [PubMed]
[16]. Slomovitz BM, Burke TW, Eifel PJ, Ramondetta LM, Silva EG, Jhingran A, Uterine Papillary Serous Carcinoma (UPSC): a single institution review of 129 casesGynecol Oncol 2003 91(3):463-69.10.1016/j.ygyno.2003.08.01814675663 [Google Scholar] [CrossRef] [PubMed]
[17]. Song T, Choi CH, Lee YY, Kim TJ, Lee JW, Kim BG, Which is worse: uterine papillary serous carcinomas or carcinosarcomas?J Gynecol Oncol 2011 22(2):83-88.10.3802/jgo.2011.22.2.8321860733 [Google Scholar] [CrossRef] [PubMed]
[18]. Gehrig PA, Bae-Jump VL, Boggess JF, Groben PA, Fowler WC Jr, Van Le L, Association between uterine serous carcinoma and breast cancerGynecol Oncol 2004 94(1):208-11.10.1016/j.ygyno.2004.04.00915262144 [Google Scholar] [CrossRef] [PubMed]
[19]. Sherman ME, Sturgeon S, Brinton LA, Potischman N, Kurman RJ, Berman ML, Risk factors and hormone levels in patients with serous and endometrioid uterine carcinomasMod Pathol 1997 10(10):963-68. [Google Scholar]
[20]. Mahdi H, Nutter B, Abdul-Karim F, Amarnath S, Rose PG, The impact of combined radiation and chemotherapy on outcome in uterine papillary serous carcinoma compared to chemotherapy aloneJ Gynecol Oncol 2016 27(2):e1910.3802/jgo.2016.27.e1926463437 [Google Scholar] [CrossRef] [PubMed]
[21]. Sherman ME, Devesa SS, Analysis of racial differences in incidence, survival, and mortality for malignant tumours of the uterine corpusCancer 2003 98(1):176-86.10.1002/cncr.1148412833470 [Google Scholar] [CrossRef] [PubMed]