A Rare Cause of Haemoptysis-Carcinoma Thyroid
Deependra Rai1
1 Assistant Professor, Department of Pulmonary Medicine, All India Institute of Medical Sciences, Patna, Bihar, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Deependra Rai, Assistant Professor, Department of Pulmonary Medicine, All India Institute of Medical Sciences, Patna-801507, Bihar, India.
E-mail: drdeependra78@gmail.com
Thyroid cancer generally metastasises to lung parenchyma while endobronchial metastasis is rare. Tracheal invasion may lead to haemoptysis or dyspnea. Most patients who present with thyroid carcinoma have well differentiated histology, having an excellent prognosis. We report a case of thyroid swelling which presented with haemoptysis as chief complaint which on investigation came out to be papillary variant of thyroid carcinoma with lung metastasis and tracheal invasion in a 46-year-old male.
Endobronchial invasion, Metastasis, Trachea
Case Report
A 46-year-old male, non-smoker, non-alcoholic presented to pulmonary medicine outpatient service with complaint of cough with blood tinged sputum since 3 month. He also had a swelling in lower neck since 1 year, which was found as thyroid swelling on examination.
The patient had normal thyroid profile. Fine Needle Aspiration Cytology (FNAC) was done from thyroid swelling before he came to our institute, which was reported as colloid goitre. There was no history of fever, appetite loss, chest pain, breathlessness or weight loss.
He was afebrile, with blood pressure of 132/68 mmHg, heart rate of 92 beats per minute and respiratory rate of 16 breaths per minute with oxygen saturation of 97% on room air. There was mild pallor with no sign of cyanosis, clubbing, and pedal oedema or lymphadenopathy. Local examination showed a 4×3 cm sized lump which was firm in consistency, mobile in the lower anterior part of neck and moving with deglutition and tongue protrusion. Chest auscultation revealed bilateral vesicular breath sounds with no added sound and in cardiovascular system examination S1S2 was present and no murmur was heard.
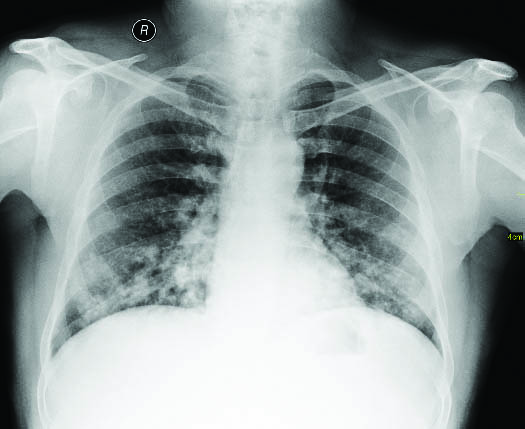
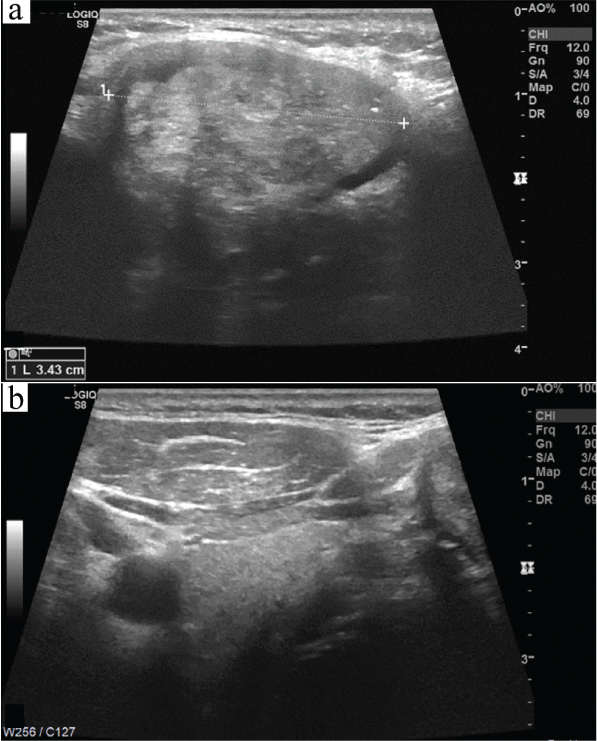
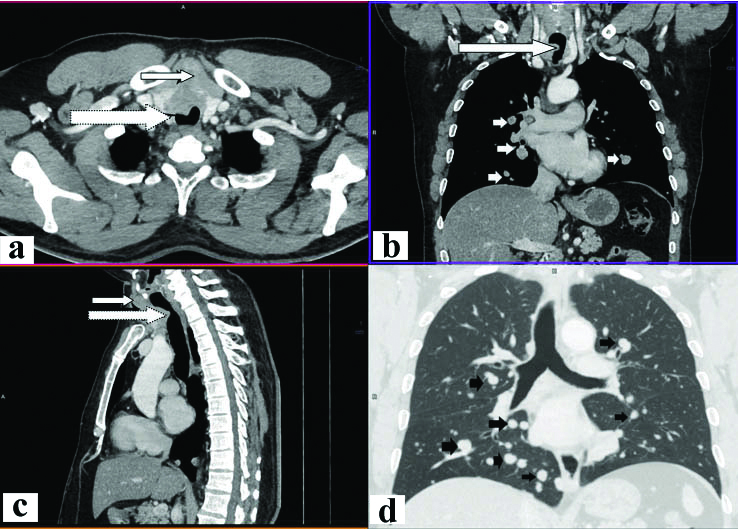
Lab Investigations showed haemoglobin of 10.5 gm%, normal kidney function test, liver function test, and serum ACE levels. Chest X-ray [Table/Fig-1] showed multiple macro-nodular opacity in bilateral lower zones. Ultrasonography of the neck [Table/Fig-2] showed a heterogeneous mass without clearly defined walls involving the thyroid gland, with some portions of the thyroid being intact and normal. No calcification was seen. Patient was further investigated by contrast enhanced Computed Tomography (CT) of thorax [Table/Fig-3] in which heterogeneous thyroid mass 5.5×4×3 cm in size in the midline was present {single short thin arrows in [Table/Fig-3a,c]} with pressure effect and invasion into trachea {single long thin arrows in [Table/Fig-3a-c]}. The mass was heterogeneously enhancing without any calcifications. Multiple cannon ball soft tissue density lesions were seen in both lungs suggestive of pulmonary metastasis [Table/Fig-3b,d]. Few small (around 8 mm in size) metastatic bilateral lower cervical (visceral space, supraclavicular and lower jugular) and upper mediastinal lymph nodes were present.
Chest radiograph, frontal view, showing extensive soft tissue density macronodules in lower zones, perihilar regions and right paracardiac region.
High resolution ultrasonography image (a) showing a heterogeneous mass (b) normal portion of thyroid.
CECT thorax study: a) axial view at lung apex showing heterogeneously enhancing mass involving the thyroid (thin arrow) invading and bulging into the trachea (thick arrow); b) coronal reformatted view at right pulmonary artery level showing the thyroid mass invading the trachea (long arrow) with multiple well defined pulmonary metastases (short arrows); c) mid-sagittal reformatted view showing the thyroid mass (thin arrow) invading and bulging into the trachea (thick arrow); d) coronal reformatted view in lung window at tracheal bifurcation level, showing multiple well defined pulmonary metastases (short arrows).
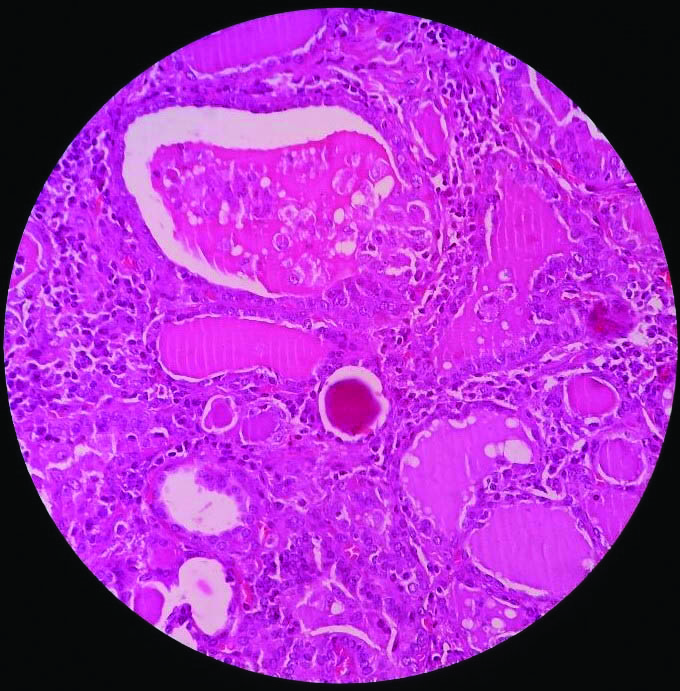
USG guided FNAC of thyroid and right cervical lymph node was performed which confirmed Papillary Carcinoma Thyroid (PCT) with metastatic nodal deposit respectively. Histology showed microscopic appearance of a papillary carcinoma showing well-formed papillae and cells with ground glass nuclei [Table/Fig-4].
Histology showed microscopic appearance of a cystic papillary carcinoma showing well-formed papillae and cells with ground glass nuclei (10X).
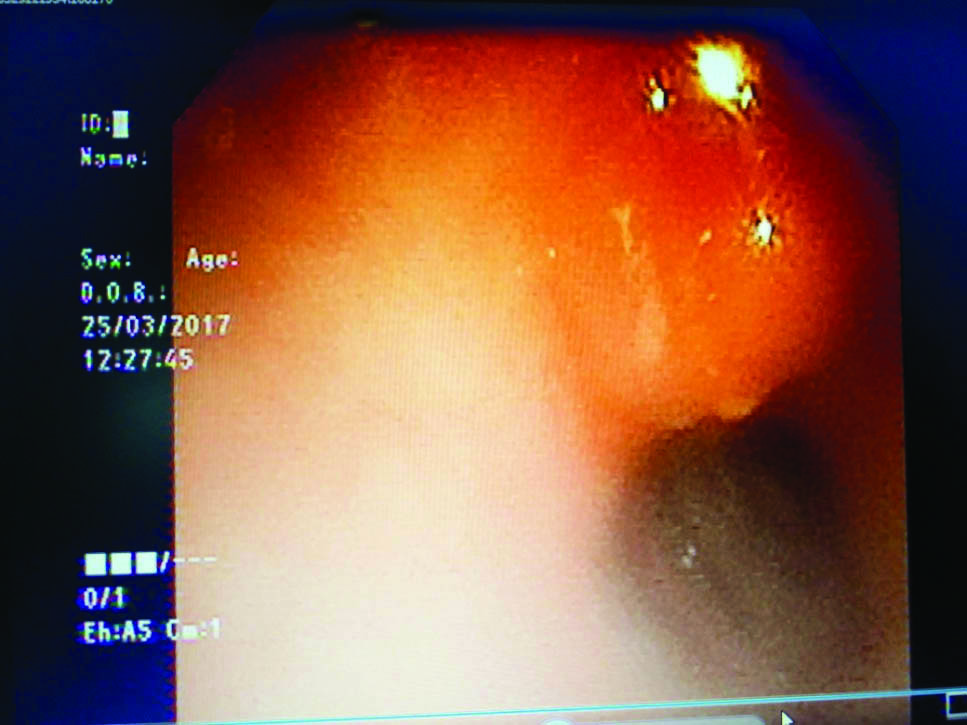
Flexible bronchoscopy was also performed which showed pressure effect over upper part of trachea just below vocal cords with friable mucosa and active blood oozing [Table/Fig-5]. Biopsy could not be tried because of heavy risk of bleeding. Tracheal aspirate was taken which was positive for malignant cells. Patient was referred to Tata memorial cancer institute, where he underwent total thyroidectomy and external beam radiotherapy. At sixth month review, patient was doing well, without any symptoms.
Bronchoscopic image showing ulcerating mass in anterior part of Trachea.
Discussion
Papillary carcinoma thyroid is known for its indolent nature and erratic behaviour. It commonly spreads through lymphatic. Vascular spread is rare, and if present, usually it is to bone, brain, lungs and soft tissue. Extra capsular spread, that is the extension of disease beyond the thyroid and into the soft tissues of neck, occurs in approximately 7-16% of patients [1]. The prognosis of patients with papillary thyroid carcinoma with lung metastases at time of diagnosis is same as for those whose lung metastases are discovered later on [2]. Tracheal invasion may complicate with haemoptysis or dyspnea and patients often die of haemorrhage or airway obstruction [3]. Tracheal infiltration is associated with impaired tumour-free survival and increased disease-specific mortality [4,5]. This case has presented with haemoptysis but no other symptoms apart from the lump, which had been managed as colloid goitre. On bronchoscopic examination we had found endotracheal ulcerating mass which was bleeding on touch and so biopsy was not possible. Correlating with CT scan findings it is apparent that there was invasion of the trachea by the mass. Apart from the local extension, there were multiple bilateral pulmonary, cervical and mediastinal lymph node metastases, hence classified as Stage IVC and managed accordingly. Pappalardo V et al., had reported a similar case of 65-year-old man presented with haemoptysis, cough, neck discomfort for two months. Subsequent physical examination showed a large fixed on the deep planes left thyroid mass. He had past 20 years history of multinodular goiter, fine-needle aspiration biopsy performed on the thyroid mass confirmed PTC. CT scan and fiberoptic assessment confirmed the invasiveness of the thyroid mass. No distant metastases were noted [6]. The risk of malignancy in MNG has not to be underestimated, and that a dominant nodule in MNG should be valued as if it were a solitary nodule in an otherwise normal gland [7].
Conclusion
We present a rare case of papillary thyroid carcinoma with tracheal invasion and multiple pulmonary metastases, diagnosed by bronchoscopy and diagnostic imaging findings. There is need to do image guided FNA for better yield of representative tissue. Follow up of thyroid swelling should be done meticulously.
[1]. Morton R, Ahmad Z, Thyroid cancer invasion of neck structures: epidemiology, evaluation, staging and managementCurrent Opinion in Otolaryngology & Head and Neck Surgery 2007 15(2):89-94.10.1097/MOO.0b013e328014734817413408 [Google Scholar] [CrossRef] [PubMed]
[2]. Hammoud Z, Mathisen D, Surgical management of thyroid carcinoma invading the tracheaChest Surgery Clinics of North America 2003 13(2):359-67.10.1016/S1052-3359(03)00018-8 [Google Scholar] [CrossRef]
[3]. Su S, Milas Z, Bhatt N, Roberts D, Clayman G, Well-differentiated thyroid cancer with aerodigestive tract invasion: long-term control and functional outcomesHead & Neck 2015 38(1):72-78.10.1002/hed.2385125204531 [Google Scholar] [CrossRef] [PubMed]
[4]. Shindo M, Caruana S, Kandil E, McCaffrey J, Orloff L, Porterfield J, Management of invasive well-differentiated thyroid cancer: an American head and neck society consensus statement: AHNS consensus statementHead & Neck 2014 36(10):1379-90.10.1002/hed.2361924470171 [Google Scholar] [CrossRef] [PubMed]
[5]. Lin S, Huang H, Liu X, Li Q, Yang A, Zhang Q, Treatments for complications of tracheal sleeve resection for papillary thyroid carcinoma with tracheal invasionEuropean Journal of Surgical Oncology (EJSO) 2014 40(2):176-81.10.1016/j.ejso.2013.12.00824388407 [Google Scholar] [CrossRef] [PubMed]
[6]. Pappalardo V, La Rosa S, Imperatori A, Rotolo N, Tanda M, Sessa A, Thyroid cancer with tracheal invasion: a pathological estimationGland Surgery 2016 5(5):541-45.10.21037/gs.2016.10.0227867870 [Google Scholar] [CrossRef] [PubMed]
[7]. Paul P, Ali K, Jasir M, Predictors of malignancy in solitary nodule thyroidInternational Surgery Journal 2017 4(2):70310.18203/2349-2902.isj20170217 [Google Scholar] [CrossRef]