Materials and Methods
The prospective case-control study was conducted in villages of northwestern India (Punjab) and northern India (Haryana) during December 2011 to February 2013. The present study was conducted at the departments of Medical Microbiology and Dermatology, Venereology and Leprosy, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. Study population was categorised as SD/D patients or healthy individuals as per the previous conducted study [4]. Inclusion criteria were based on the presence of inflammation of the scalp in the form of red, scaly and flaking rash. Cases applying topical antifungals and/or steroid to the scalp preceding a month of sampling were excluded from the study. Specimen was collected using a blunt scalpel from relatively severely affected areas and vertex region of SD/D patients and healthy individuals scalp respectively. The specimen collection was carried out at convenience by random screening. The degree of SD/D severity was graded as mild, moderate and severe based on size, colour and surface of the flakes/scales [9]. All isolates were identified based on phenotypic and molecular technique as described earlier [10]. Total 30 clinical isolates of Malassezia isolated from SD/D patient’s lesional area and from HC were randomly selected and included in the study. Isolates include M. globosa, n=10 (5 SD/D, 5 HC); M. restricta, n=10 (5 SD/D, 5 HC) and M. furfur, n=10 (5 SD/D, 5 HC). The study protocol was approved by the Institute Ethics Committee of Postgraduate Institute of Medical Education and Research, Chandigarh, India. The samples were collected after obtaining informed consent of the subjects. All strains were grown on Leeming and Notman Agar (LNA) media and incubated at 34°C for seven days.
Each Malassezia isolate (20 CFU/mL) was cultured on five sets of LNA plates under the condition described above. Malassezia cells were scrapped from the surface of LNA plate and transferred to prewieghed eppendorf tube. The wet weight of Malassezia cells was noted. After scraping the Malassezia cells from the surface of agar medium, the surface was rinsed twice with distilled water. LNA medium was crushed in pestle-mortar. The resultant slurry was incubated in 100 mL protein extraction buffer at 4°C for 24 hour. The mixture was filtrated through Whatman filter paper followed by 0.2 mm membrane filter (Millipore Corporation, MA, USA). The resultant solution was concentrated using Amicon Ultra centrifugal filter, ultracel- 30K (Millipore corporation, MA, USA) [5].
Lipase activity was measured using an assay based on hydrolysis of p-Nitrophenylpalmitate (pNPP). Due to low solubility in water, pNPP (Sigma, MO, USA) was first dissolved in 2-propanol at 10 mM concentration then 1 mM pNPP was dissolved in 10 mM phosphate buffer (pH=6) and 1% TritonX-100. To this substrate, 30 μL of concentrated extracted extracellular protein was added and incubated at 37°C for one hour. Before measuring the absorbance at 405 nm, 200 mL of 1 M Tris buffer (pH=8.0) was added to stabilise the pH-dependent dye pNP (p-Nitrophenol). Release of pNP from pNPP was measured in an ELISA reader as the increase in absorption at 405 nm [11].
Statistical Analysis
Comparison of lipase activity among M. globosa, M. restricta and M. furfur was analysed by ANOVA (Tukey’s multiple comparisons) test and calculated using the GraphPad Prism version 6.01 (www.graphpad.com). p<0.05 was considered as statistically significant.
Results
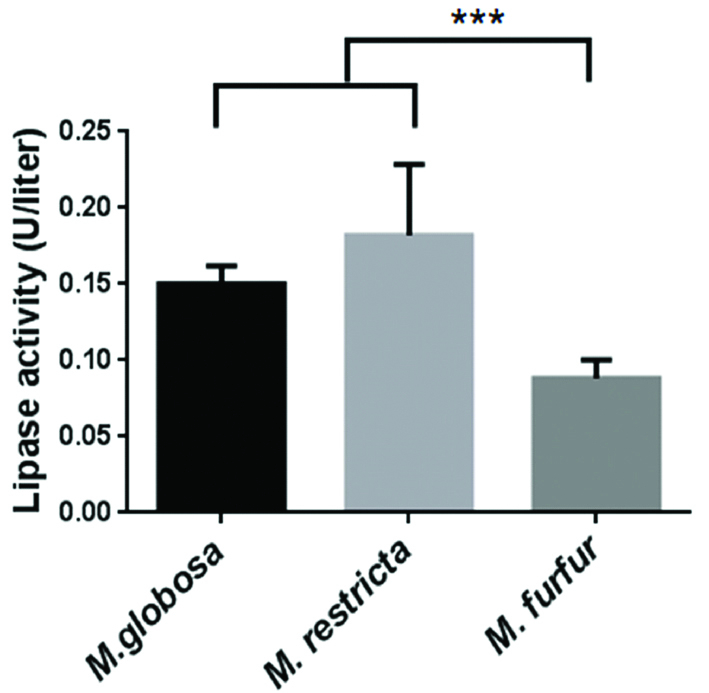
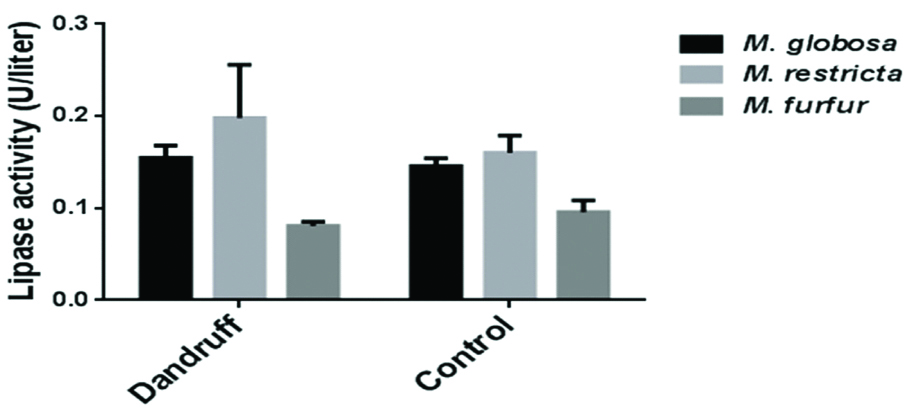
After seven days of incubation on LNA media, M. furfur average wet fungal weight was higher (1.1±0.13 g), compared to M. globosa (0.36±0.4 g) and M. restricta (0.26±0.4 g) [Table/Fig-1]. The protein concentration was normalised, as the wet weight of M. furfur was ~3 and ~2 times higher than M. globosa and M. restricta. One unit of lipase activity is defined as the amount of enzyme that released 1 μM of p-nitrophenol per minute [11]. The mean extracellular lipase activity of M. globosa (0.15 U) and M. restricta (0.18 U) was significantly higher than M. furfur (0.08 U) isolates (p<0.0001) [Table/Fig-2,3]. No significant difference in the extracellular lipase activity was observed among M. globosa (SD/D-0.15 U vs. HC-0.14 U; p=0.5818), M. restricta (SD/D-0.2 U vs. HC-0.16 U; p=0.0671) and M. furfur (SD/D-0.08 U vs. HC-0.1 U; p=0.4067) isolated from SD/D patient’s lesional area and HC [Table/Fig-4]. As there was no statistical difference between isolates from SD/D patients and HC, degree of SD/D and lipase activity was not calculated.
Isolation source and wet weight (in grams) of different Malassezia isolates.
| M. globosa | M. restricta | M. furfur |
|---|
| SD/D patients | Weight in gm | Healthy controls | Weight in gm | SD/D patients | Weight in gm | Healthy controls | Weight in gm | SD/D patients | Weight in gm | Healthy controls | Weight in gm |
| U10 | 0.44 | NU11 | 0.38 | U54 | 0.23 | NU55 | 0.32 | U5 | 1.12 | NU5 | 1.25 |
| U66 | 0.36 | NU68 | 0.38 | U64 | 0.28 | NU63 | 0.29 | U60 | 1.14 | NU9 | 1.35 |
| U71 | 0.38 | NU70 | 0.43 | U65 | 0.28 | NU65 | 0.32 | U62 | 1.32 | NU33 | 1.2 |
| U77 | 0.37 | NU75 | 0.45 | U73 | 0.25 | NU78 | 0.3 | U82 | 1.34 | NU35 | 1.28 |
| U60 | 0.39 | NU60 | 0.47 | U74 | 0.30 | NU80 | 0.27 | U89 | 1.3 | NU62 | 1.29 |
Abbreviation: SD/D, Seborrhoeic dermatitis/dandruff
U and NU areis just the lab number in which U indicates SD/D patient isolate and NU indicates HC isolates
Statistical analysis of the mean extracellular lipase activity of M. globosa, M. restricta and M. furfur isolates.
| Tukey’s multiple comparisons test | Mean Diff. | 95% CI of diff. | p-value |
|---|
| M. globosa vs. M. restricta | -0.03187 | 0.06466 to 0.0009227 | ns |
| M. globosa vs. M. furfur | 0.06181 | 0.03025 to 0.09337 | <0.001 |
| M. restricta vs. M. furfur | 0.09368 | 0.05924 to 0.1281 | <0.0001 |
Test employed: ANOVA (Tukey’s multiple comparisons) test. p<0.05 was considered as statistically significant
Lipase activity in extracted extracellular proteins of different Malassezia species. Error bars indicate the standard deviations of the background-subtracted values for three independent replicate experiments (* p<0.05- significant).
Lipase activity in extracted extracellular proteins of Malassezia species isolated from seborrhoeic dermatitis/dandruff patient’s lesional area and healthy controls.
Error bars indicate the standard deviations of the background-subtracted values for three independent replicate experiments.
Discussion
The pathobiology of Malassezia in SD/D is not clear, due to the complex interaction of the commensal yeast with the skin. Malassezia shows lipid dependency due to the absence of fatty acid synthase gene. Malassezia yeast secretes the extracellular lipolytic enzymes to obtain saturated fatty acids from the environment [8]. The lipolytic enzymes (lipase, phospholipase, esterase and lysophospholipase) may be associated with the virulence of Malassezia in SD/D.
Juntachai W et al., compared the extracellular lipase activity of standard strains of seven Malassezia spp. M. globosa (CBS 7986) had the highest lipase activity compared to the other six Malassezia spp. (M. pachydermatis, M. restricta, M. furfur, M. slooffiae, M. sympodialis and M. obtusa) [5]. However, there are no relative reports regarding secreted lipase activities of Malassezia species associated with SD/D. In the present study the lipase activity of M. globosa and M. restricta was higher despite slow growth rate (the wet weight of species indicate the growth rate; higher the wet weight higher the growth rate) contrasting the results of previous study [5]. This difference may be due to source of isolation. In the previous study the standard strains were used to compare the lipase activity, whereas in the present study the isolates were from SD/D patients and HC [5]. This is the first in-vitro study to investigate the lipase activity between the Malassezia strains isolated from SD/D patient’s lesional area and healthy controls. Recently Park M et al., characterised MrLip1, lipase gene of M. restricta, isolated from a SD patient. However, it would have been interesting to characterise expression of this gene in M. restricta isolated from healthy individual [12].
Ran Y et al., for the first time studied the role of lipase and its significance in M. furfur. The lipase was detected in the insoluble fraction of the cells and was acidic in nature. Association between cell growth and lipase activity was observed [13]. Brunke S et al., in 2006 cloned and characterised MfLIP1 (gene encoding extracellular lipase of M. furfur) [11]. Conserved lipase motif of C. albicans has shown to be similar to MfLIP1. In the same year, Shibata N et al., cloned and characterised M. pachydermatis extracellular lipase gene [14]. But both M. furfur and M. pachydermatis have least association in the causation of SD/D. Many reports including the previous study have shown that M. globosa and M. restricta are the species most commonly associated with SD/D [9]. Even the whole genome sequence of M. globosa has revealed the presence of 14 lipase-encoding genes, of which 13 were predicted secreted proteins [8]. However, only two genes (MgLIP1 and MgLIP2) have been isolated and characterised [15,16]. It also contained conserved lipase motif. Late log phase of M. globosa expressed higher MgLIP1. Due to fastidious nature of M. restricta, its lipase activity was not detected. In addition, recently MgLIP1 expression was detected on the human scalp [17].
In the present study, in a given species no difference has been observed in the lipase activity among the strains isolated from lesional area of SD/D patients and HC. These results suggest that lipase activity alone may not play a role in the onset of SD/D. However, lipase may help in metabolising lipids and integrating the products on cell wall leading to growth and survival in the environment. As the pathogenic mechanism of fungi is complex, it is difficult to explain the pathogenicity of Malassezia based on lipase activity alone. Other virulence factors of Malassezia species may play a role in pathogenesis either alone or in combinations. The activity of phospholipase is an important virulence that has been detected in M. pachydermatis and implicated in the occurrence of skin lesions [18]. In addition, secreted phospholipase activities of M. furfur were detected in vitro by the egg-yolk method [19]. Quantitative analysis and comparison of phospholipase activities of M. globosa and M. restricta isolated from SD/D patient’s lesional area and HC may provide more insight on the pathogenesis of SD/D.
Limitation
The limitation of present study was the low number of Malassezia strains studied, due to difficulty in cultivating these fastidious species. Due to small number of strains we couldn’t comment on the degree/severity of SD/D and lipase activity. Storage of the Malassezia strains for longer period may have reduced the enzyme activity.
Conclusion
Despite slow growth rate of M. globosa and M. restricta, the lipase activity was higher than M. furfur. No difference was observed in the lipase activity among the strains isolated from lesional area of SD/D patients and HC. Malassezia pathogenesity is a complex mechanism. Hence, lipase activity alone may not play a role in the causation of SD/D.
Abbreviation: SD/D, Seborrhoeic dermatitis/dandruff
U and NU areis just the lab number in which U indicates SD/D patient isolate and NU indicates HC isolates
Test employed: ANOVA (Tukey’s multiple comparisons) test. p<0.05 was considered as statistically significant