Many studies have been reported along the DFU patients who had been commonly encountered with predominant pathogens such as P. aeruginosa, E. coli, Staphylococcus spp., Klebsiella spp., Acinetobacter spp. and Proteus spp. Only few of them reported DFU and non-DFU patients with A. baumannii infections with threatening drug-resistant and limb amputation [7].
Materials and Methods
A hospital-based, prospective study was conducted over a period of 10 months from September 2016 to June 2017 at a Tertiary Care Hospital, Mangalore, Karnataka, India, after obtaining Institutional Ethics Committee approval (Reg. No- YU2016/172). Informed consent was obtained from all the participants.
Study design and sample collection: Samples (pus and exudates, tissues) were collected from patients who presented with foot ulcer infections. A total of 70 non-duplicate isolates were obtained from 400 patients screened. Thirty five isolates of A. baumannii were taken in each, among DFU and non-DFU patients. Inclusion criteria for DFU group was patient having DFU infection with age >18 years. Duplicate sample of the same patient were excluded. Inclusion criteria for non-DFU group included patient having ulcer with peripheral arterial disease, neuropathy and age >18 years. Patients with history of prior treatment and Meggitt Wagner classification system grading of foot ulcer: 0–I were excluded [17].
Bacteria Isolation and Identification
The collected pus/tissues were processed for Gram staining and cultured on 5% Sheep blood agar and MacConkey agar (Hi-Media Laboratories, India) for aerobic culture and incubated at 37°C overnight. Tissues were homogenised before inoculation and all isolates were confirmed using the BD Phoenix 100 system (Becton Dickinson, USA).
Antimicrobial Susceptibility Testing (AST)
AST was carried out by Kirby-Bauer method on Mueller Hinton Agar (MHA) and the results were interpreted according to CLSI 2016 guidelines [18]. Antibiotic disks tested were amikacin (30 μg), imipenem (10 μg), meropenem (10 μg), piperacillin (100 μg), piperacillin/tazobactam (110 μg), levofloxacin (5 μg), ciprofloxacin (5 μg), co-trimoxazole (Trimethoprim/sulfamethoxazole) (1.25/23.75 μg), ceftriaxone (30 μg), cefotaxime (30 μg), ceftazidime (30 μg), cefepime (30 μg), gentamicin (10 μg), tobramycin (10 μg) and tetracycline (30 μg) (Hi-Media Laboratories, India). For control, E. coli (ATCC® 25922™), E. coli (ATCC® 35218™) and P. aeruginosa (ATCC® 27853™) were used.
Minimum Inhibitory Concentration (MIC)
The MIC of colistin was determined for all the MDR A. baumannii isolates by using the E-test strips and agar methods according to the manufacturer’s instructions. The MIC breakpoints of ≤2 μg/L were regarded as susceptible and ≥4 μg/L as resistant [18,19].
Extended Spectrum of β-Lactamase (ESBL)
Detection of the ESBL phenotype was performed by combined disk diffusion method on MHA plate using ceftazidime (30 μg), ceftazidime/clavulanic acid (30/10 μg) [18]. E. coli (ATCC® 25922™) were used as the negative control and an in-house ESBL producing A. baumannii (ATCC® 19606™) isolate was used as the positive control. The test was considered positive when an increase in the diameter of the zone of inhibition was ≥5 mm around ceftazidime/clavulanic acid against ceftazidime alone [19-21].
Metallo-β-Lactamase (MBL)
Detection of MBL was done by using Imipenem-EDTA combined disk test. The overnight broth cultures of test isolates along with standard control strains (opacity adjusted to 0.5 McFarland) was lawn cultured on MHA plate. After drying, 10 μg imipenem disks were placed on the lawn culture with 20 mm distance from the centre to centre of the disks. Another 10 μg imipenem impregnated with 750 μg of disodium EDTA was added to one of the imipenem disks and incubated overnight [18]. Isolates which showed ≥7 mm more inhibition zone (size) of imipenem – EDTA disk than compared to the imipenem disk alone, were considered as MBL producers. Positive control used was P. aeruginosa (ATCC® 27853™) [22,23].
Modified Hodge Test (MHT)
Modified Hodge test was performed in all isolates. It is a screening test which helps in detection of carbapenemases. E. coli (ATCC® 25922™) an indicator organism sensitive to carbapenems was cultured in peptone water to achieve 0.5 McFarland opacity standard and lawn cultured onto an MHA plate using a sterile cotton swab [18]. After drying, 10 μg imipenem disk was placed at the centre of the plate on the lawn culture, and an overnight growth of the test strain was heavily streaked from the edge of the imipenem disk outwards, to the periphery of the plate in one direction. After streaking positive and negative control from the edge of the disk outwards to the periphery of the plates, they were incubated at 37°C overnight and the presence of a distorted zone - clover-leaf shaped zone of inhibition was considered as a positive test [24,25].
Biofilm Formation Assay
Biofilm forming ability of all the isolates was performed as mentioned previously with certain modification [26]. Briefly, bacterial cells were grown overnight at 37°C in 5 mL Trypticase-Soy Broth (TSB) in a test tube. A 96-well flat bottomed polystyrene tissue culture plate was added with 200 μL of sterile TSB, inoculated with 10 μL of overnight culture and incubated at 37°C for 24 hours. After incubation, layer from each well was removed and washed carefully three times, with 200 μL of phosphate buffered saline (pH-7.2) in order to remove free-floating bacteria. Adherent bacteria were added with 200 μL of 99% methanol for 15 minutes. The plates were decanted, dried and stained for 7 minutes with 200 μL of 0.1% Hucker crystal violet. Excess stain was rinsed off with tap water. The plates were air dried and dye bound to the adherent cells was solubilised with 160 μL of 33% (v/v) glacial acetic acid per well. The Optical Density (OD) of each well was measured at 630 nm using ELISA reader (FLUOstar Omega, BMG LABTECH, Germany). The biofilm producing strain A. baumannii (ATCC® 19606™) and P. aeruginosa (ATCC® 27853™) were taken for Positive Control (PC). Wells inoculated with sterile broth were used as Negative Control (NC).
Statistical Analysis
Statistical analysis was performed using SPSS software version 23.0 (SPSS Inc., Chicago, IL). The chi-square test and Fisher’s-exact two-tailed test analysis were done in this study. Statistical significance was regarded as a p-value < 0.05.
Results
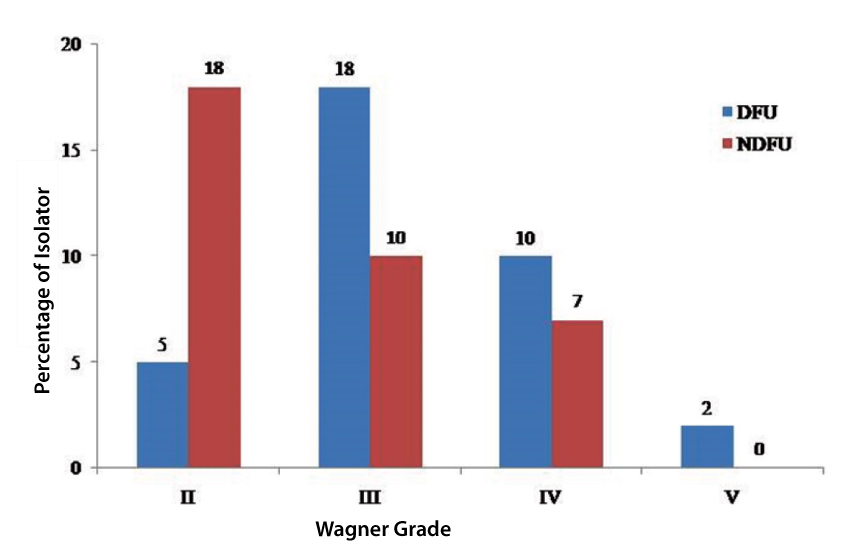
Out of the 70 isolates of A. baumannii obtained from 400-foot ulcer patients (17.50%), 35 (15.90%) isolates (35/220) were recovered from DFU and 35 (19.44%) isolates (35/180) from non-DFU patients. The mean age group of the patients was 56.57+10.34 years. Most of the isolates were recovered from the foot ulcer patients who were 44 to 60 years old [Table/Fig-1] and Wagner grading II to V [Table/Fig-2]. Infections on males were predominant 77.14% (27/35) compared to females 28.86% (8/35) among DFU and non-DFU patients. In present study, DFU patients were suffering from associated diseases such as hypertension, neuropathy, retinopathy, and nephropathy. Few patients also presented with ischaemic heart diseases. In non-DFU patients below the age of 30, had history of smoking/alcohol or drinking and above the age of 30, in addition to this had hypertension.
Distribution of age group and sex affected with DFU and NDFU in Acinetobacter baumannii infections.
| Age (years) | DFU (35/220=15.90%) | NDFU (35/180=19.44%) |
|---|
| Male | Female | Male | Female |
|---|
| 18-30 | 2 | 1 | 5 | 3 |
| 31-43 | 3 | 1 | 8 | 1 |
| 44-56 | 8 | 4 | 7 | 1 |
| ≥57 | 14 | 2 | 7 | 3 |
| Total | 27 | 8 | 27 | 8 |
DFU: Diabetic foot ulcer; NDFU: Non-diabetic foot ulcer
Isolate distribution in different grades of ulcer in DFU and non-DFU patients.
Antimicrobial susceptibility pattern of A. baumannii among DFU and non-DFU patients is depicted in [Table/Fig-3]. All the DFU patients isolates of A. baumannii were resistant to major groups of antibiotics in comparison to non-DFU. DFU were sensitive to 100% colistin and resistant to ceftazidime (97.14%), cefotaxime (94.29%), 91.43% (cefepime, co-trimoxazole, and tetracycline), 88.57% (amikacin, ceftriaxone, ciprofloxacin, piperacillin), gentamicin (85.71%), 82.56% (levofloxacin, imipenem, piperacillin/tazobactam), tobramycin (80%) and meropenem (77.14%). NDFU were sensitive to 51.43% (amikacin, cefepime, gentamicin, tobramycin, piperacillin/tazobactam), 54.29% (ceftriaxone, imipenem), levofloxacin (57.14%), meropenem (68.57%), 100% colistin and resistant to 65.71% (tetracycline, ceftazidime, ciprofloxacin), cefotaxime (60%), and 51.43% (piperacillin, co-trimoxazole).
Antimicrobial susceptibility pattern of Acinetobacter baumannii in DFU and NDFU.
| Antibiotic disc | Acinetobacterbaumannii* | Sensitive | Resistant | p-value* |
|---|
| AMK | DFU | 4 (11.43%) | 31 (88.57%) | 0.00062 |
| NDFU | 18 (51.43%) | 17 (48.57%) |
| CPM | DFU | 3 (8.57%) | 32 (91.43%) | 0.000167 |
| NDFU | 18 (51.43%) | 17 (48.57%) |
| CAZ | DFU | 1 (2.86%) | 34 (97.14%) | 0.000678 |
| NDFU | 12 (34.29%) | 23 (65.71%) |
| CTX | DFU | 2 (5.71%) | 33 (94.29%) | 0.001208 |
| NDFU | 14 (40.00%) | 21 (60.00%) |
| CTR | DFU | 4 (11.43%) | 31 (88.57%) | 0.000144 |
| NDFU | 19 (54.29%) | 16 (45.71%) |
| CIP | DFU | 4 (11.43%) | 31 (88.57%) | 0.026617 |
| NDFU | 12 (34.29%) | 23 (65.71%) |
| LEV | DFU | 6 (17.14%) | 29 (82.56%) | 0.001093 |
| NDFU | 20 (57.14%) | 15 (42.86%) |
| COT | DFU | 3 (8.57%) | 32 (91.43%) | 0.000402 |
| NDFU | 17 (48.57%) | 18 (51.43%) |
| GEN | DFU | 5 (14.29%) | 30 (85.71%) | 0.001907 |
| NDFU | 18 (51.43%) | 17(48.57%) |
| IPM | DFU | 6 (17.14%) | 29 (82.56%) | 0.002409 |
| NDFU | 19 (54.29%) | 16 (45.71%) |
| MRP | DFU | 8 (22.86%) | 27 (77.14%) | 0.000144 |
| NDFU | 24 (68.57%) | 11 (31.43%) |
| PIP | DFU | 4 (11.43%) | 31 (88.57%) | 0.000402 |
| NDFU | 17 (48.57%) | 18 (51.43%) |
| PIT | DFU | 6 (17.14%) | 29 (82.56%) | 0.005056 |
| NDFU | 18 (51.43%) | 17 (48.57%) |
| TET | DFU | 3 (8.57%) | 32 (91.43%) | 0.010239 |
| NDFU | 12 (34.29%) | 23 (65.71%) |
| TOB | DFU | 7 (20%) | 28 (80%) | 0.011862 |
| NDFU | 18 (51.43%) | 17 (48.57%) |
Notes: *Fisher exact two-tailed test (p-value < 0.05 and significant difference between DFU and non-DFU patients for all antibiotics).
*Acinetobacter baumannii isolated from diabetic and non-diabetic foot ulcer patients (Tissue, Pus and exudates)
AMK: Amikacin; CPM: Cefepime; CAZ: Ceftazidime; CTX: Cefotaxime; CTR: Ceftriaxone; CIP: Ciprofloxacin; LEV: Levofloxacin; COT: Co-trimoxazole; GEN: Gentamicin; IPM: Imipenem; MRP: Meropenem; PIP: Piperacillin; PIT: Piperacillin/tazobactam; TET: Tetracycline; TOB: Tobramycin; DFU: Diabetic foot ulcer; NDFU: Non-diabetic foot ulcer
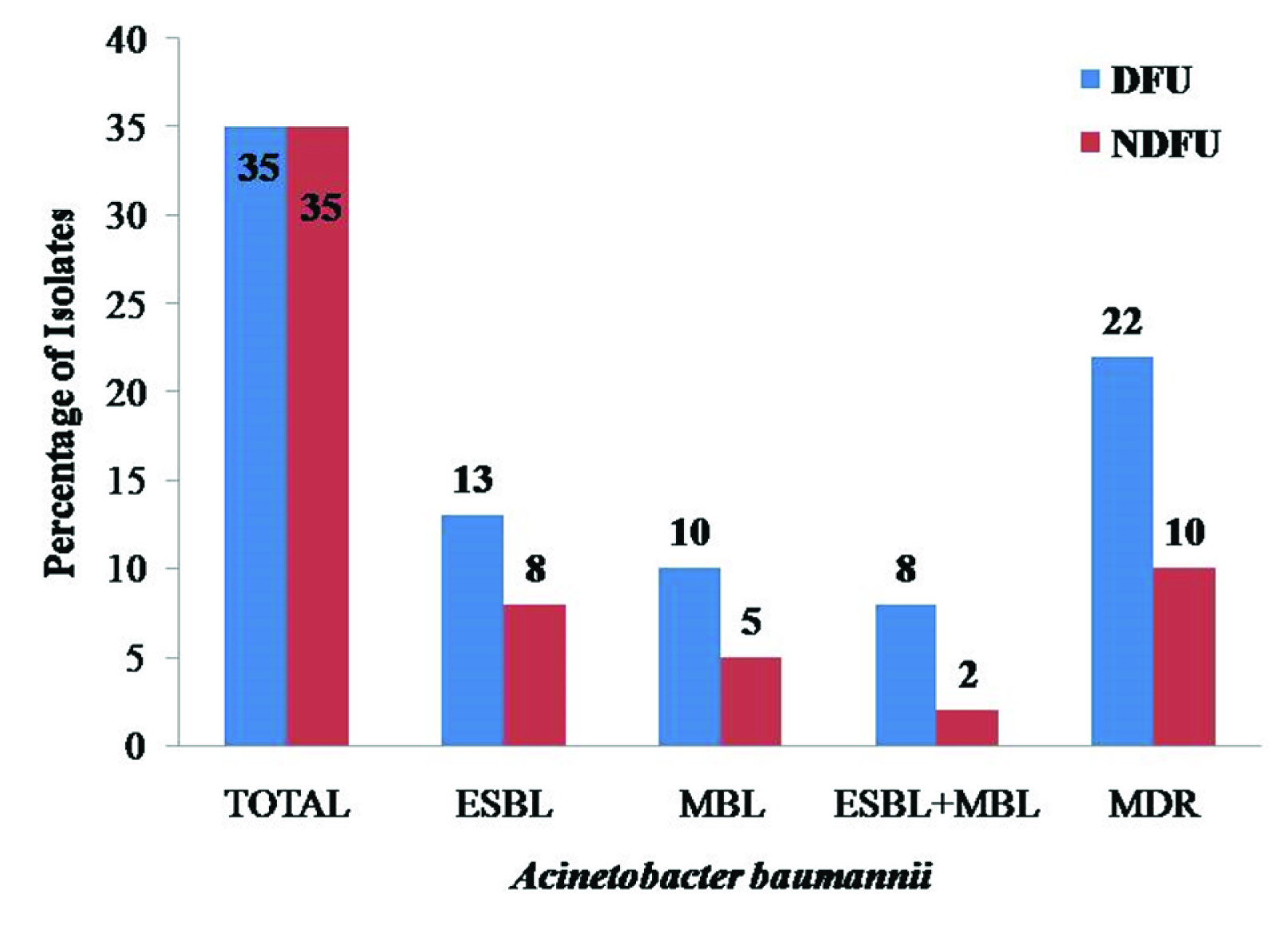
ESBL and MBL producing isolates among A. baumannii were 37.14% (13/35) and 28.57% (10/35). Non-DFU isolates were ESBL 22.85% (8/35), and MBL 14.28% (5/35) respectively. All isolates of DFU and non-DFU isolates which are MBL positive were confirmed by MHT. MDR isolates of A. baumannii were 62.85% (22/35) in DFU and 28.57% (10/35) in non-DFU patients [Table/Fig-4]. Biofilm formation was seen in A. baumannii from DFU and more compared to non-DFU isolates [Table/Fig-5a,b]. Based on the tissue culture plate assay, the isolates were classified as strong {Optical Density (OD) >0.350}, moderate (OD between 0.200-0.350) or weak (OD 0.041-0.200) biofilm formation in A. baumannii. A chi-square (χ2) test showed that there was a significant difference between isolates from DFU and non-DFU patients with A. baumannii infections {p-value <0.05 (0.034); χ2 value=6.71 and degree of freedom (df)=2} which correlated with ESBL, MBL production and MDR with biofilm formation [Table/Fig-6a,b]. The hospital stays of patients in whom ESBL, MBL producer, biofilm formation, and MDR were isolated ranged between 15-40 days.
Percentage of ESBL, MBL producer and MDR Acinetobacter baumannii.
Comparison of biofilm formation in Acinetobacter baumannii among DFU and non-DFU isolates.
| Biofilm Formation | DFU (n=35) | NDFU (n=35) | p-value |
|---|
| Weak (0.041 – 0.200) | 03 (8.57%) | 10 (28.57%) | χ2=6.71, df=2, p=0.034 |
| Moderate (≥0.200 – 0.350) | 23 (65.71%) | 22 (62.86%) |
| Strong (≥0.350) | 09 (25.72%) | 03 (8.57%) |
Chi-square (χ2) test; Significant p-value: <0.05, df: Degree of freedom; DFU: Diabetic foot ulcer; NDFU: Non-diabetic foot ulcer
Biofilm formation in Acinetobacter baumannii in DFU and NDFU isolates.
PC: Positive control, NC: Negative control; DFU: Diabetic foot ulcer; NDFU: Non-diabetic foot ulcer

Correlation of ESBL, MBL and MDR Acinetobacter baumannii with Biofilm formation in DFU
| Acinetobacter baumannii | Biofilm Formation-DFU |
|---|
| Strong (09/35) | Medium(23/35) | Weak(03/35) |
|---|
| ESBL | 08 | 05 | 0 |
| MBL | 08 | 02 | 0 |
| MDR | 09 | 11 | 02 |
ESBL: Extended spectrum of β-lactamase; MBL: Metallo-β-lactamase; MDR: Multi-drug resistant; DFU: Diabetic foot ulcer
Correlation of ESBL, MBL and MDR Acinetobacter baumannii with Biofilm formation in NDFU.
| Acinetobacter baumannii | Biofilm Formation-NDFU |
|---|
| Strong (03/35) | Medium (22/35) | Weak (10/35) |
|---|
| ESBL | 02 | 05 | 01 |
| MBL | 02 | 03 | 0 |
| MDR | 03 | 07 | 0 |
ESBL: Extended spectrum of β-lactamase; MBL: Metallo-β-lactamase; MDR: Multi-drug resistant; NDFU: Non-diabetic foot ulcer
Discussion
Foot ulcer infection complications are frequent clinical problems among non-communicable diseases leading to hospitalisation. Most commonly foot ulcer infection seen in patients who tend to have immune deficiency, mono-microbial, and poly-microbial infections. Foot ulcer infections are predominantly poly-microbial with the ability to form changing trends of susceptibility, ESBL, MBL, MDR and biofilm, are important causative agents resulting in treatment failure and increased risk of amputation.
This study shows A. baumannii infections in 15.90% DFU and 19.44% non-DFU patient, which is slightly higher than Murali TS et al., study which reported 14% in both isolates [13]. Another similar study by Karmaker M et al., reported the prevalence of 10% Acinetobacter spp. in DFU and non-DFU infections [27]. El-Din RA et al., reported 33% of A. baumannii isolated from DFU patients [15]. Jyothylekshmy V et al., reported the prevalence of 5.3% Acinetobacter spp. in DFU patients [28]. In present study, DFU isolates were 100% sensitive to colistin and resistant to ceftazidime (97.14%), cefotaxime (94.29%), 91.43% (cefepime, co-trimoxazole, and tetracycline), 88.57% (amikacin, ceftriaxone, ciprofloxacin, piperacillin), gentamicin (85.71%), 82.56% (levofloxacin, imipenem, piperacillin/tazobactam), tobramycin (80%) and meropenem (77.14%). Mendes JJ et al., reported sensitivity to 100% colistin and resistant to all routine antibiotics in DFU isolates [16]. Shanmugam P and Jeya M, reported antibiotic resistant were 83% ceftazidime, 67% ciprofloxacin, 50% (cefepime, gentamicin, tetracycline, tobramycin), 33% (amikacin, cotrimoxazole), 17% (imipenem, piperacillin/ tazobactam) in DFU isolates [7]. Another similar study, done by Murali TS et al., [13] reported DFU isolates resistant to amikacin (76%), cefotaxime (94%), ciprofloxacin (84%), and gentamicin (90%). Acinetobacter spp. were resistant to cefotaxime (88%), gentamicin (59%), amikacin (53%), ciprofloxacin (36%), and 29% (ceftazidime, piperacillin, piperacillin/tazobactam) in DFU isolates [29]. Similarly, Turhan V et al., in their study reported Acinetobacter spp. were resistant to piperacillin/tazobactam (88%), ciprofloxacin (84%), co-trimoxazole (75%), ceftazidime (63%), amikacin (53%), imipenem (29%) [30]. Akhi MT et al., reported antibiotic resistance to 100% (tetracycline, cefepime, ceftriaxone), and 50% (gentamicin, ciprofloxacin, imipenem, piperacillin/tazobactam) [31]. Results of present study indicated a significant difference between in DFU and non-DFU patients for all isolates, p-value <0.05 [Table/Fig-3].
Non-DFU patient’s isolates were susceptible to 100% colistin, meropenem (68.57%), levofloxacin (57.14%), 54.29% (ceftriaxone, imipenem), 51.43% (amikacin, cefepime, gentamicin, tobramycin, piperacillin/tazobactam) and resistant to 65.71% (tetracycline, ceftazidime, ciprofloxacin), cefotaxime (60%), and 51.43% (piperacillin, co-trimoxazole). Murali TS et al., reported antibiotic sensitivity to non-DFU isolates were 25% (amikacin, ciprofloxacin, gentamicin) and resistance to 100% cefotaxime, and 75% (amikacin, ciprofloxacin) [13].
ESBL and MBL production in A. baumannii was reported on many studies but unfortunately, CLSI guidelines do not support or advocate. A. baumannii producing ESBL was first reported from Turkey in 2001 and India in 2007. Similarly, MBL production in A. baumannii was reported from South Korea in 2001 and India in 2011. Few studies are reported on diabetic foot ulcer infection in A. baumannii. This study shows that among DFU isolates, 37.14% (13/35) A. baumannii were ESBL producer and MBL 28.57% (10/35). Shanmugam P et al., reported 33.33% were ESBL and 16.6% MBL producers [7]. Another study by El-Din RA et al., reported 34.61% MBL producers [15]. The present study showed that, non-DFU isolates were producing ESBL 22.85% (8/35) and 14.28% (5/35) MBL. Surprisingly, none of the studies has reported ESBL and MBL production in non-DFU isolates of A. baumannii. Some studies has reported, but it is not clear whether specimens/sample should be taken from the patient of peripheral arterial and peripheral neuropathy or not. Hence, this study is first which reports ESBL and MBL production in A. baumannii among non-DFU.
Almost all MDR isolates of A. baumannii produce biofilm in DFU and non-DFU patients. Biofilm formation was categorised into strong, moderate, and weak. In this study 25.72% (9/35), 65.71% (23/35), 8.57% (03/35) prevalence was reported as strong, moderate and weak respectively. A significant difference was observed between diabetic and non-diabetic groups [Table/Fig-5a,b]. Similar to our study, Swarna SR and Gomathi S, reported 23.07% strong biofilm formation and 15.38% moderate biofilm formation which was much lower than the biofilm formation observed in present study and 38.46% weak which was higher compared to our study [32]. Murali TS et al., reported biofilm formation in DFU isolates was 39.13% [13]. Another study by Zubair M et al., reported 60% strong and 40% weak biofilm formation in DFU isolates [33]. Similarly, Vatan A et al., reported 59.25% strong and 40.75% weak biofilm formation [34]. Di Domenico EG et al., reported 8.9% strong biofilm formation in DFU isolates [35].
Biofilm formation in non-DFU patients of A. baumannii in present study showed 8.57% (03/35) strong, 62.86% (22/35) moderate, and 28.57% (10/35) weak biofilm formation. Amazingly, we found that biofilm formation in DFU isolates correlating with ESBL, MBL and MDR A baumannii were more predominant than non-DFU isolates [Table/Fig-6a,b]. Murali TS et al., reported the correlation of biofilm and MDR isolates in DFU and non-DFU patients that were more virulent compared to non-DFU isolates leading to limb amputation [13]. In present study, we found wide variation and changing trend of antimicrobial susceptibility pattern compared to other studies. DFU patients compared to non-DFU patients had significant difference of healing ulcer due to their compromised immune status. There is a need to understand the characteristics of A. baumannii in non-healing ulcer infections among DFU and non-DFU isolates.
Limitation
In the present study, the MIC of netilmicin and polymixin B was not determined as the duration of sample collection was limited. The study did not include any molecular approach to find out ESBL, MBL, and drug resistance genes.
Conclusion
This study highlights the need to establish antimicrobial susceptibility surveillance for A. baumannii to determine the appropriate empirical treatment regimen. Periodical checkup in a tertiary care hospital with the help of infection control and wound/ulcer management committee will help to inhibit the spreading of A. baumannii infections. The biofilm formation in DFU compared to non-DFU patients is the most severe threat to the non-healing ulcers. The rapid spreading of ESBL, MBL producers, and MDR require the implementation of not just surveillance study but also a proper and rational selection of antibiotics, especially for MDR which help the clinician in the treatment of diabetic and non-DFU infection patients.
DFU: Diabetic foot ulcer; NDFU: Non-diabetic foot ulcer
Notes: *Fisher exact two-tailed test (p-value < 0.05 and significant difference between DFU and non-DFU patients for all antibiotics).
*Acinetobacter baumannii isolated from diabetic and non-diabetic foot ulcer patients (Tissue, Pus and exudates)
AMK: Amikacin; CPM: Cefepime; CAZ: Ceftazidime; CTX: Cefotaxime; CTR: Ceftriaxone; CIP: Ciprofloxacin; LEV: Levofloxacin; COT: Co-trimoxazole; GEN: Gentamicin; IPM: Imipenem; MRP: Meropenem; PIP: Piperacillin; PIT: Piperacillin/tazobactam; TET: Tetracycline; TOB: Tobramycin; DFU: Diabetic foot ulcer; NDFU: Non-diabetic foot ulcer
Chi-square (χ2) test; Significant p-value: <0.05, df: Degree of freedom; DFU: Diabetic foot ulcer; NDFU: Non-diabetic foot ulcer
ESBL: Extended spectrum of β-lactamase; MBL: Metallo-β-lactamase; MDR: Multi-drug resistant; DFU: Diabetic foot ulcer
ESBL: Extended spectrum of β-lactamase; MBL: Metallo-β-lactamase; MDR: Multi-drug resistant; NDFU: Non-diabetic foot ulcer