Andrographolide, A Novel Repressor of Hepcidin Gene Expression
Vadoud Malekzadeh1, Farideh Manafi2, Reza Alipanah-Moghadam3, Ali Nemati4, Arash Mehri5, Firouz Norouzi6, Mohammad Mohammadzadeh-Vardin7, Firouz Amani8
1 Instructor, Department of Anatomical Sciences, Research Laboratory for Embryology and Stem Cells, School of Medicine, Ardabil University of Medical Sciences, Ardabil, Iran.
2 Master of Science, Department of Clinical Biochemistry, School of Medicine, Ardabil University of Medical Sciences, Ardabil, Iran.
3 Assistant Professor, Department of Clinical Biochemistry, School of Medicine, Ardabil University of Medical Sciences, Ardabil, Iran.
4 Associate Professor, Department of Clinical Biochemistry, School of Medicine, Ardabil University of Medical Sciences, Ardabil, Iran.
5 Master of Science, Department of Clinical Biochemistry, School of Medicine, Ardabil University of Medical Sciences, Ardabil, Iran.
6 Master of Science, Department of Clinical Biochemistry, School of Medicine, Ardabil University of Medical Sciences, Ardabil, Iran.
7 Assistant Professor, Department of Anatomical Sciences, Research Laboratory for Embryology and Stem Cells, School of Medicine, Ardabil University of Medical Sciences, Ardabil, Iran.
8 Associate Professor, Department of Social Medicine, School of Medicine, Ardabil University of Medical Sciences, Ardabil, Iran.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Reza Alipanah-Moghadam, Daneshgah Street, Ardabil University of Medical Sciences, Ardabil, Iran.
E-mail: alipanahreza9@gmail.com
Introduction
Hepcidin is the most important factor in iron metabolism and plays a potential role in erythropoiesis. It is a small peptide hormone which is cysteine-rich and is mostly secreted by hepatic cells.
Aim
The purpose of this study was to evaluate the effect of andrographolide on the expression of hepatic hepcidin in an iron overload model.
Materials and Methods
For the current study, 48 male Wistar rats were used in six groups. These groups included control group, andrographolide with 3.5 and 7 mg/kg doses groups, iron plus andrographolide groups with 3.5 and 7 mg/kg doses, and iron group. Hepcidin gene expression was performed by real-time method. Iron serum levels were measured by photometry. We used ANOVA and Kruskal Wallis to compare the means of the factors under investigation.
Results
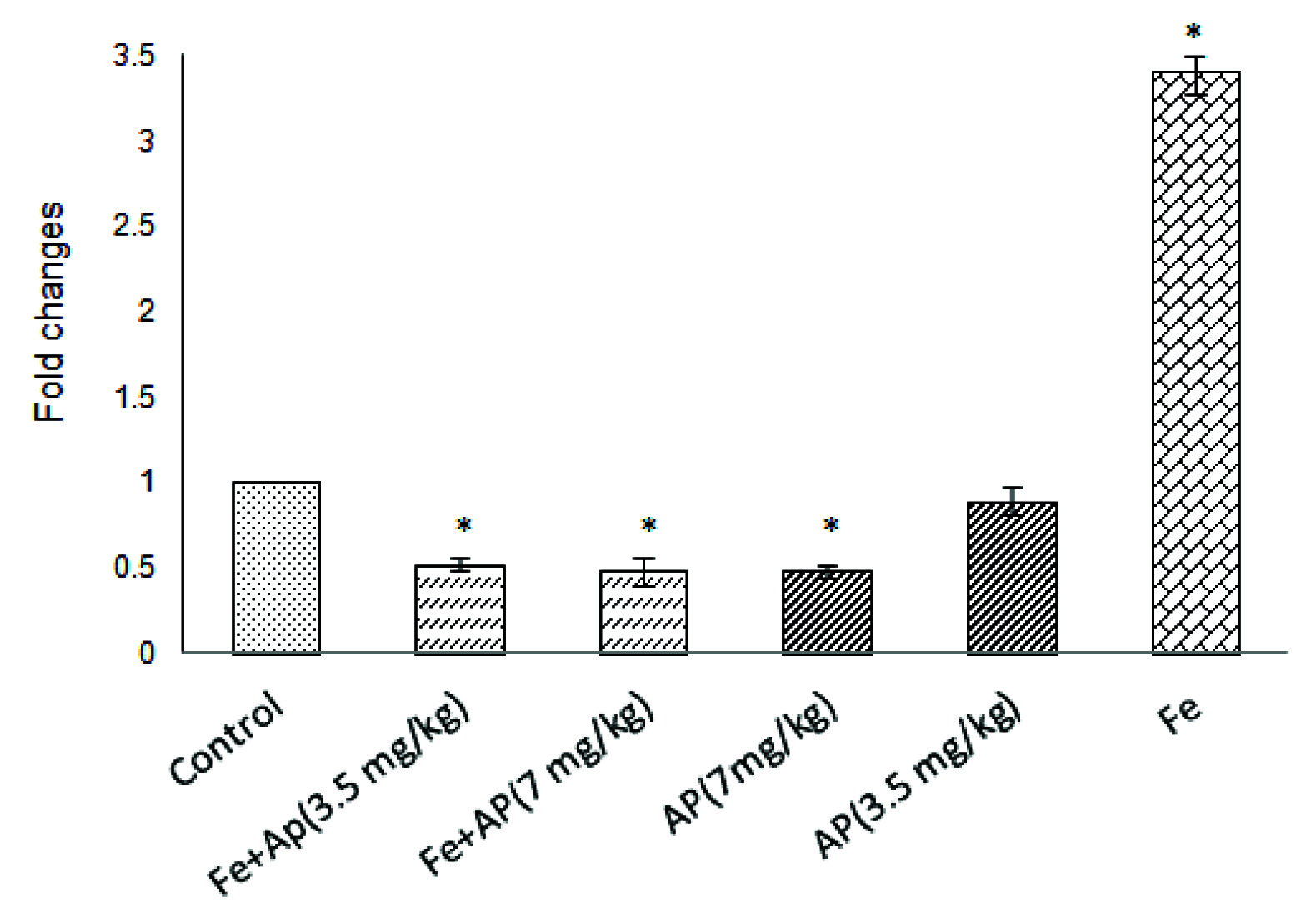
The results revealed that the quantitative expression levels of mRNA hepcidin decreased in all groups except in iron group compared with the control group. This decrease in andrographolide 7 mg/kg, iron plus andrographolide 3.5 mg/kg, and iron plus andrographolide 7 mg/kg groups was significant compared to the control group (p<0.05). The quantitative expression level of mRNA hepcidin significantly increased in iron group as compared to the control group (p<0.05). The findings also indicated that serum concentration of iron in groups with secondary iron overload significantly increased compared with the control group (p<0.05).
Conclusion
Andrographolide with 3.5 and 7 mg/kg doses decreases the expression of hepcidin and increases iron serum levels in secondary iron overload model.
Anaemia, Andrographis paniculata, Iron Overload
Introduction
Hepcidin is a one of the factors that most recently has been recognized and plays an important role in the regulation of erythropoiesis. It is a small peptide hormone which is cysteine-rich and is mostly secreted by hepatic cells. Hepcidin plays a vital role in constant maintenance of iron homeostasis in the human body. Its identification at the beginning of the new millennium has brought a new perspective on the understanding of the iron metabolism. Hepcidin controls absorption of dietary iron by connecting to enterocytes of duodenal. In addition, by connecting to macrophages, it inhibits the release of iron. Intestinal iron absorption, iron uptake, and its release from cell resources and finally haematopoiesis is controlled by hepcidin [1,2]. It has been demonstrated that removal of hepcidin gene can cause severe forms of iron overload disease [3]. On the contrary, high expression of hepcidin results in the decrease of iron absorption and iron deficiency anaemia [4].
One of the most important inducers of hepcidin expression is chronic inflammation which can lead to anaemia [5]. The treatment of anaemia caused by chronic inflammation is an important emergency for the patients with this condition. It has been proven that increased expression of hepcidin plays an important role in the development of anaemia in patients with chronic inflammation [6]. One of the medicinal herbs that have potent anti-inflammatory effects is Andrographis paniculata. This plant is the acanthaceae family that contains diterpanes, lactones and flavonoids. Andrographolide is the most effective isolated agent from Andrographis paniculata. It has been shown that androgrophloide has a very effective role in inhibiting inflammatory processes and increase the expression of heme oxygenase 1, and in this way, it interferes with iron recycling [7-10]. In this study, we hypothesized that iron overload would increase hepatic hepcidin expression, so we examined the effect of andrographolide on hepatic hepcidin expression.
Materials and Methods
The present experimental study was conducted with permission from the Animal Ethics Committee of Ardabil University of Medical Sciences, in December 2016 (Ethical code: IR.ARUMS.REC.1394.125). In this study, 48 adult male Wistar strain rats weighing approximately 100-150 g were used. The rats were kept in standard conditions of temperature and food.
The rats were randomly divided into six groups of eight: 1) The control group (receiving 1 cc of physiologic serum as intraperitoneal injection); 2) receiving iron sucrose group (75 mg/kg body weight); 3) receiving 3.5 mg/kg andrographolide group; 4) receiving 7 mg/kg andrographolide group; 5) receiving iron sucrose 75 mg/kg plus 3.5 mg/kg andrographolide group; and 6) receiving iron sucrose 75 mg/kg plus 7 mg/kg andrographolide group. To create iron overload, iron receiving groups received iron sucrose (Venofer-Germany) for two weeks with a dose of 75 mg per kilogram of body weight.
Andrographolide was purchased in the form of powder from Sigma Company (365645-100MG). It was prepared in PBS solution at concentrations of 3.5 and 7 mg and was injected intraperitoneal in a volume of 1 ml. The experimental period to create iron overload was six days. After the sixth day, the rats were anesthetized and blood samples were taken from the heart and then they were killed. Then, their livers were removed and cut into smaller pieces and were kept in Falcon tubes at -80°C until the experiment.
Tissue samples were also fixed in 10% formalin for histology. Liver samples were embedded in paraffin for staining to examine iron deposition. Slides were mounted with 4 μm sections stained with haematoxylin & eosin, and examined by light microscopy by an experienced pathologist. To extract RNA from the hepatic tissue, Trizol (Thermo Fisher Scientific, USA) was used. In this method, 100 mg of hepatic tissue was homogenized, and then 1000 μl Trizol was added. To separate the RNA from protein and DNA phases, the chloroform solution was used. To precipitate the RNA, a solution of isopropanol was employed. All the equipment and materials were RNAse Free. RNA quality and quantity were measured using the NanoDrop spectrophotometer (Thermo Scientific- USA) and RNA integrity was determined by gel electrophoresis. To ensure that there was no contamination of the RNA, DNAse I enzyme was employed. In this method, 1 μl of the extracted RNA was mixed with 1 μl buffer of TBE 10X and 0.5 μl of DNase I (1u/μl), and the final volume was brought to 10 μl using RNAse Free DEPC water. Then the samples were kept for 30 minutes at 37°C. Then about 1 μl of EDTA 50 mM was added to all samples and it was incubated for 10 minutes at 65°C. For the synthesis of cDNA from the extracted RNA, Sina Gen standard kit (RT-5201) was employed. To prepare Master mix for the Real-time PCR reaction of hepcidin and GAPDH genes, 12.5 μl SYBR green, 0.5 μl forward primer, 0.5 μl reverse primer, and 1 μl cDNA were mixed together. The final volume with Nuclease free water (NF water) was brought to 20 μl. The temperature cycle was set as follows: First 10 minutes at 95°C, followed by 40 cycles at 94°C for 30 seconds, 30 seconds at 60°C, 30 seconds at 72°C, 10 seconds at 95°C, 60 seconds at 65°C, 1 second at 97°C, and 30 seconds at 37°C. Primers of [Table/Fig-1] were used for the Real-time PCR of hepcidin and GAPDH (as controller) genes. Serum iron levels were measured using a standard kit and photometry method.
The sequences of primers in hepcidin and GAPDH genes.
| Gene | Forward primer(5’ → 3’) | Reverse primer(5’ → 3’) | Tm (°C) | Ampliconsize (bp) |
|---|
| Hepcidin | AAGATGGCACTA | GCATTTACAGCA | 60 | 219 |
| GAPDH | GTTACCAGGGCT | GGGTTTCCCGTT | 60 | 110 |
Real-time PCR data analysis was conducted using 2-ΔΔCT method. In this method, the rate of change in hepcidin gene expression was calculated using GAPDH gene as the reference gene.
Data Analysis
We used ANOVA and Kruskal Wallis (SPSS Ver. 16) to compare the means of the factors under investigation. The level of significance was set at p<0.05.
Results
The results revealed that the quantitative expression levels of mRNA hepcidin decreased in all groups except in iron group compared with the control group [Table/Fig-2]. This decrease in andrographolide 7 mg/kg, iron plus andrographolide 3.5 mg/kg, and iron plus andrographolide 7 mg/kg groups was significant compared with the control group (p<0.05). The quantitative expression level of mRNA hepcidin significantly increased in iron group compared with the control group (p<0.05).
Comparison of hepcidin gene expression levels in different studied groups.
* Existence of statistically significant difference compared with the control group (p<0.05)
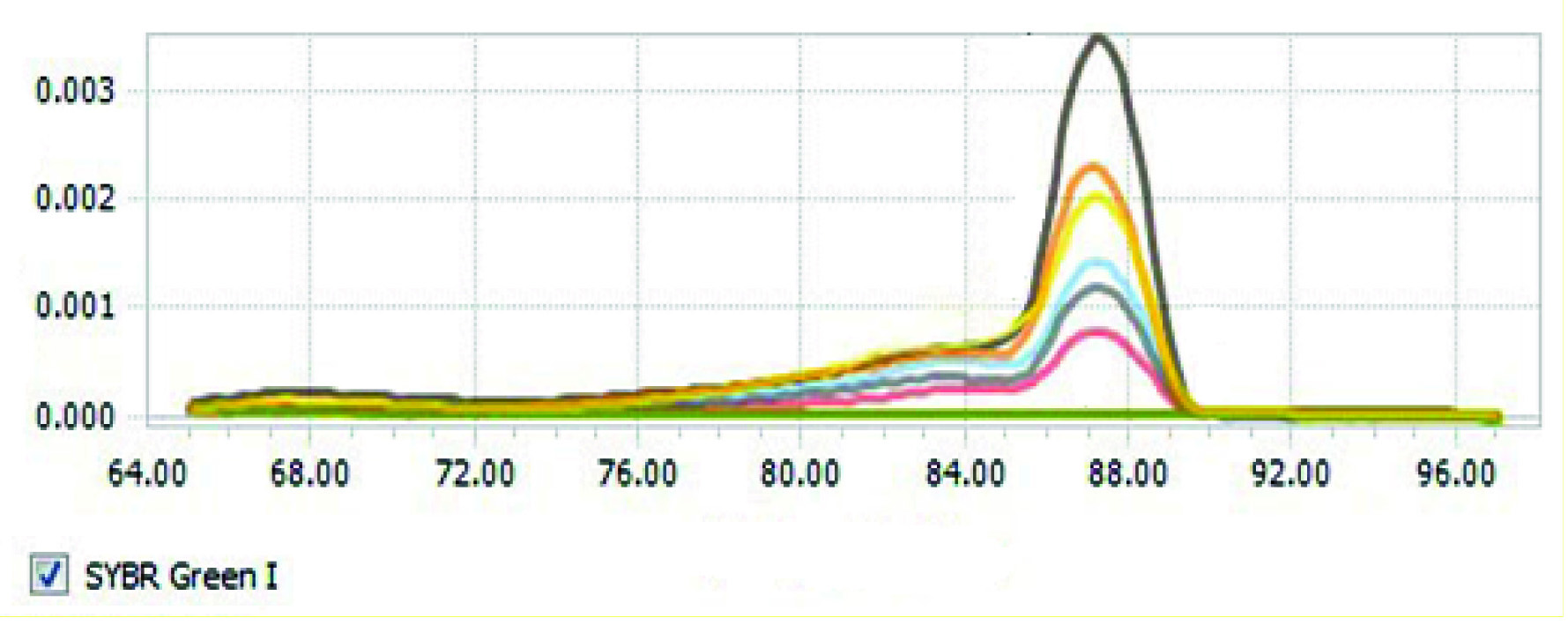
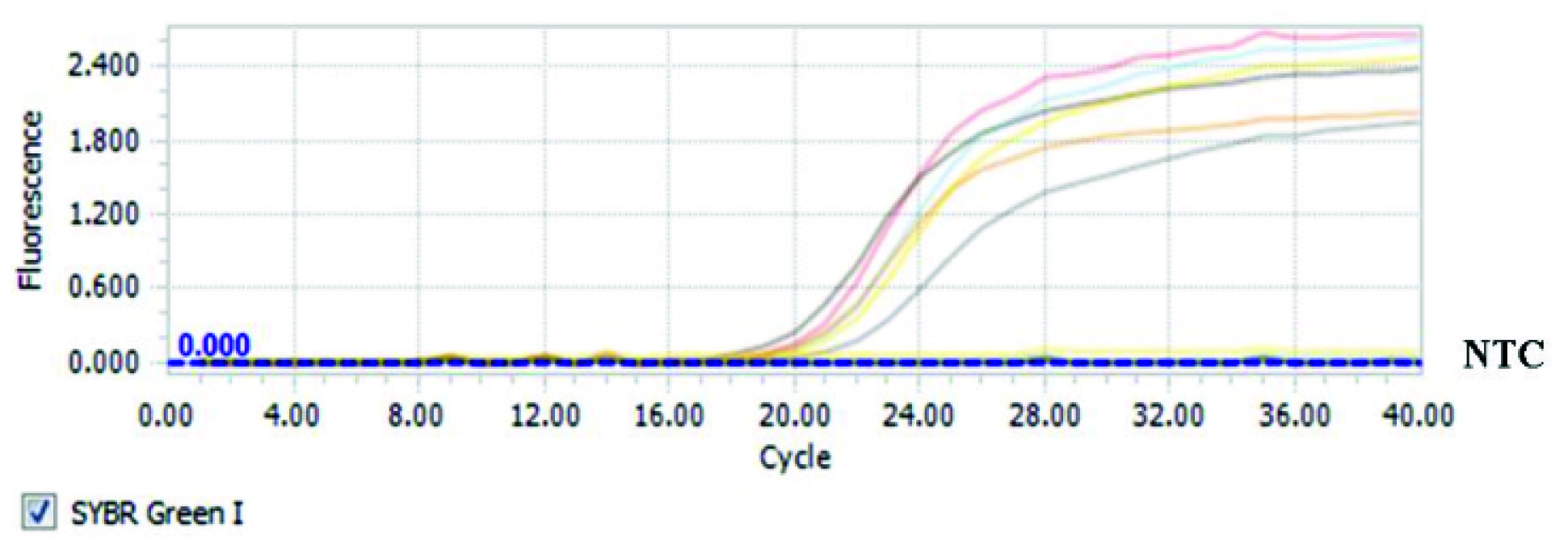
The findings also indicated that serum concentration of iron in groups with secondary iron overload significantly increased compared with the control group (p<0.05) [Table/Fig-3]. Increased serum levels of iron in the iron plus andrographolide 7 mg/kg and iron plus andrographolide 3.5 mg/kg groups were more significant. Hepatic tissue samples of rats with iron overload compared with the control group have been shown in [Table/Fig-4]. The results of the melting curve for hepcidin gene in different groups have been shown in [Table/Fig-5]. The results of the threshold cycle for hepcidin gene in different groups have been given in [Table/Fig-6].
Mean serum iron concentration in control and different studied groups.
** Existence of statistically significant difference compared with the control group (p<0.001)

Hepatic tissue samples in control group (a) and rats with iron overload (b).
* The arrows show iron deposition.

The melting curve of hepcidin gene in different studied groups.
The threshold cycle of hepcidin gene in different studied groups.
Discussion
The results of our findings revealed that the quantitative expression levels of mRNA hepcidin decreased in all groups except in iron group compared with the control group. Hepcidin is one of the hormones that have recently attracted the attention of many researchers. As the main regulator of iron distribution and by controlling ferroportin on the surface of intestinal cells and macrophages, it limits the supply of iron for haematopoiesis. In addition to its effect on reducing iron supply for haematopoiesis, it also has some inhibitory effects on haematopoiesis of the bone marrow [5,11,12]. Hepcidin also decreases the proliferation and the survival of progenitor red blood cells [13]. Increasing in hepcidin serum levels is considered as one of the main causes of anaemia and a decrease in haemoglobin levels in patients with chronic inflammation [14]. Since curing anaemia in these patients is one of the medical priorities [15,16], so investigating the factors that affect hepcidin expression is necessary. In this study, we have tried to create a model of secondary iron overload as hepatic hepcidin expression inducer to investigate the effect of andrographolide on enhanced hepatic hepcidin expression. We showed that andrographolide can inhibit expression levels of mRNA hepcidin in iron overload animal model. Therefore, andrographolide can be used to improve the anaemia of inflammatory diseases.
In our study, by injecting iron sucrose, the quantitative hepcidin mRNA expression in iron overload group significantly increased compared with the control group. This finding was in accordance with several other related studies [17,18]. It has been demonstrated that increased expression of hepcidin is considered body’s defence mechanism to prevent the intestinal iron absorption and iron overload in the body [18]. In our study, decreased hepatic hepcidin mRNA expression was observed in all injected groups with andrographolide at doses of 3.5 and 7 mg/kg compared with the control and iron groups. Reduced hepcidin expression by andrographolide injection in groups with secondary iron overload was much higher than those who were just affected by andrographolide without secondary iron overload. Basically, the existence of iron overload is results in increased hepcidin expression compared with samples without iron overload [19]. Our results also confirmed this finding and showed that hepcidin expression levels had strong relationship with iron serum levels. As with reduced expression of hepcidin levels resulted in increasing serum iron levels. In iron overload groups affected by andrographolide increasing in iron serum levels was more than just iron overload group. It can be caused by reduced expression of hepcidin in these groups. Andrographolide is a diterpene combination extracted from the Andrographis paniculata plant [20].
In most studies, anti-inflammatory and antioxidant effects of andrographolide have been investigated. Studies have revealed that andrographolide inhibits the production of inflammatory markers of IL-12, TNF-α, NF-κB, iNOS and COX-2 in macrophages. Furthermore, andrographolide inhibits the inflammatory process through covalent binding to the transcription factor of NF-kB and by forming complex with NF-kB prevents it from binding to DNA [21-24]. There is little research regarding the effects of andrographolide on the expression of some genes. It has been shown that andrographolide by reducing the synthesis and the stability of nitric oxide synthase inhibits this enzyme in post-translational processes [22]. Also, andrographolide or its derivatives can increase the expression of CYP1A1, CYP1A2 and CYP1B1 genes, but decrease the expression of TNF [23,25]. In addition, andrographolide with intracellular increasing of NRF2, can increase the expression of heme oxygenase 1, and in this way, it interferes with iron recycling [9,10]. According to many studies, andrographolide inhibits the expression of IL-6 as one of the most important markers of inflammation [26-28]. Since IL-6 is one of the most important stimuli in hepcidin production, so increase in the amount of IL-6 in chronic inflammatory conditions can increase the production of hepcidin [29,30]. Therefore, andrographolide by inhibiting the production of IL-6 reduce the production of hepcidin. In our study, probably a total of factors such as andrographolide effects in reducing the inflammatory process, or its effect on hepcidin expression via IL-6 or its impact on the processes of post-translational protein have been involved. Our study is the first step in andrographolide effects on hepcidin gene expression and more research should be done in this field.
Conclusion
Andrographolide at doses of 3.5 and 7 mg/kg reduces hepcidin expression and increases serum levels of iron in secondary iron overload model.
[1]. Vokurka M, Krijt J, Sulc K, Necas E, Hepcidin mRNA levels in mouse liver respond to inhibition of erythropoiesisPhysiol Res 2006 55(6):667-74. [Google Scholar]
[2]. Nemeth E, Ganz T, Regulation of iron metabolism by hepcidinAnnu Rev Nutr 2006 26:323-42.10.1146/annurev.nutr.26.061505.11130316848710 [Google Scholar] [CrossRef] [PubMed]
[3]. Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, The gene encoding the iron regulatory peptide hepcidin is regulated by anaemia, hypoxia, and inflammationJ Clin Invest 2002 110(7):1037-44.10.1172/JCI021568612370282 [Google Scholar] [CrossRef] [PubMed]
[4]. Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, Severe iron deficiency anaemia in transgenic mice expressing liver hepcidinProceedings of the National Academy of Sciences 2002 99(7):4596-601.10.1073/pnas.07263249911930010 [Google Scholar] [CrossRef] [PubMed]
[5]. Ganz T, Hepcidin, a key regulator of iron metabolism and mediator of anaemia of inflammationBlood 2003 102(3):783-88.10.1182/blood-2003-03-067212663437 [Google Scholar] [CrossRef] [PubMed]
[6]. Andrews NC, Anaemia of inflammation: the cytokine-hepcidin linkJ Clin Invest 2004 113(9):1251-53.10.1172/JCI2144115124013 [Google Scholar] [CrossRef] [PubMed]
[7]. Chao W-W, Lin B-F, Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian)Chinese Medicine 2010 5(1):01-15.10.1186/1749-8546-5-1720465823 [Google Scholar] [CrossRef] [PubMed]
[8]. Zaheer M, Giri CC, Enhanced diterpene lactone (andrographolide) production from elicited adventitious root cultures of Andrographis paniculataResearch on Chemical Intermediates 2017 43(4):2433-44.10.1007/s11164-016-2771-9 [Google Scholar] [CrossRef]
[9]. Lee JC, Tseng CK, Young KC, Sun HY, Wang SW, Chen WC, Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/Nrf2 pathway in human hepatoma cellsBritish Journal of Pharmacology 2014 171(1):237-52.10.1111/bph.1244024117426 [Google Scholar] [CrossRef] [PubMed]
[10]. Poss KD, Tonegawa S, Heme oxygenase 1 is required for mammalian iron reutilizationProceedings of the National Academy of Sciences 1997 94(20):10919-24.10.1073/pnas.94.20.109199380735 [Google Scholar] [CrossRef] [PubMed]
[11]. Singh B, Arora S, Agrawal P, Gupta S, Hepcidin: a novel peptide hormone regulating iron metabolismClinica Chimica Acta 2011 412(11-12):823-30.10.1016/j.cca.2011.02.01421333642 [Google Scholar] [CrossRef] [PubMed]
[12]. Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalizationScience 2004 306(5704):2090-93.10.1126/science.110474215514116 [Google Scholar] [CrossRef] [PubMed]
[13]. Dallalio G, Law E, Means RT, Hepcidin inhibits in vitro erythroid colony formation at reduced erythropoietin concentrationsBlood 2006 107(7):2702-04.10.1182/blood-2005-07-285416332970 [Google Scholar] [CrossRef] [PubMed]
[14]. Niihata K, Tomosugi N, Uehata T, Shoji T, Mitsumoto K, Shimizu M, Serum hepcidin-25 levels predict the progression of renal anaemia in patients with non-dialysis chronic kidney diseaseNephrology Dialysis Transplantation 2012 27(12):4378-85.10.1093/ndt/gfs32222833619 [Google Scholar] [CrossRef] [PubMed]
[15]. De Nicola L, Minutolo R, Conte G, Anaemia management in non-dialysis chronic kidney disease: flexibility of target to target stability?Nephron Clin Pract 2010 114(4):c236-41.10.1159/00027657420090364 [Google Scholar] [CrossRef] [PubMed]
[16]. Lahera V, Goicoechea M, de Vinuesa SG, Oubiña P, Cachofeiro V, Gómez-Campderá F, Oxidative stress in uremia: the role of anaemia correctionJournal of the American Society of Nephrology 2006 17(12 suppl 3):S174-S77.10.1681/ASN.200608091117130258 [Google Scholar] [CrossRef] [PubMed]
[17]. Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout miceProceedings of the National Academy of Sciences 2001 98(15):8780-85.10.1073/pnas.15117949811447267 [Google Scholar] [CrossRef] [PubMed]
[18]. Papanikolaou G, Tzilianos M, Christakis JI, Bogdanos D, Tsimirika K, MacFarlane J, Hepcidin in iron overload disordersBlood 2005 105(10):4103-05.10.1182/blood-2004-12-484415671438 [Google Scholar] [CrossRef] [PubMed]
[19]. Camaschella C, Iron and hepcidin: a story of recycling and balanceASH Education Program Book 2013 2013(1):01-08.10.1182/asheducation-2013.1.124319154 [Google Scholar] [CrossRef] [PubMed]
[20]. Zhao F, He E-Q, Wang L, Liu K, Anti-tumor activities of andrographolide, a diterpene from Andrographis paniculata, by inducing apoptosis and inhibiting VEGF levelJournal of Asian Natural Products Research 2008 10(5):467-73.10.1080/1028602080194833418464090 [Google Scholar] [CrossRef] [PubMed]
[21]. Bao Z, Guan S, Cheng C, Wu S, Wong SH, Kemeny DM, A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-κB pathwayAmerican Journal of Respiratory and Critical Care Medicine 2009 179(8):657-65.10.1164/rccm.200809-1516OC19201922 [Google Scholar] [CrossRef] [PubMed]
[22]. Chiou WF, Chen CF, Lin JJ, Mechanisms of suppression of inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells by andrographolideBritish Journal of Pharmacology 2000 129(8):1553-60.10.1038/sj.bjp.070319110780958 [Google Scholar] [CrossRef] [PubMed]
[23]. Qin L-H, Kong L, Shi G-J, Wang Z-T, Ge B-X, Andrographolide inhibits the production of TNF-α and interleukin-12 in lipopolysaccharide-stimulated macrophages: role of mitogen-activated protein kinasesBiological and Pharmaceutical Bulletin 2006 29(2):220-24.10.1248/bpb.29.22016462022 [Google Scholar] [CrossRef] [PubMed]
[24]. Lee K-C, Chang H-H, Chung Y-H, Lee T-Y, Andrographolide acts as an anti-inflammatory agent in LPS-stimulated RAW264. 7 macrophages by inhibiting STAT3-mediated suppression of the NF-κB pathwayJournal of Ethnopharmacology 2011 135(3):678-84.10.1016/j.jep.2011.03.06821497192 [Google Scholar] [CrossRef] [PubMed]
[25]. Jaruchotikamol A, Jarukamjorn K, Sirisangtrakul W, Sakuma T, Kawasaki Y, Nemoto N, Strong synergistic induction of CYP1A1 expression by andrographolide plus typical CYP1A inducers in mouse hepatocytesToxicology and Applied Pharmacology 2007 224(2):156-62.10.1016/j.taap.2007.07.00817825862 [Google Scholar] [CrossRef] [PubMed]
[26]. Lim JCW, Chan TK, Ng DS, Sagineedu SR, Stanslas J, Wong W, Andrographolide and its analogues: versatile bioactive molecules for combating inflammation and cancerClinical and Experimental Pharmacology and Physiology 2012 39(3):300-10.10.1111/j.1440-1681.2011.05633.x22017767 [Google Scholar] [CrossRef] [PubMed]
[27]. Chun JY, Tummala R, Nadiminty N, Lou W, Liu C, Yang J, Andrographolide, an herbal medicine, inhibits interleukin-6 expression and suppresses prostate cancer cell growthGenes & Cancer 2010 1(8):868-76.10.1177/194760191038341621442031 [Google Scholar] [CrossRef] [PubMed]
[28]. Kou W, Sun R, Wei P, Yao H-B, Zhang C, Tang X-Y, Andrographolide suppresses IL-6/Stat3 signaling in peripheral blood mononuclear cells from patients with chronic rhinosinusitis with nasal polypsInflammation 2014 37(5):1738-43.10.1007/s10753-014-9902-524803294 [Google Scholar] [CrossRef] [PubMed]
[29]. Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidinThe Journal of Clinical Investigation 2004 113(9):1271-76.10.1172/JCI20042094515124018 [Google Scholar] [CrossRef] [PubMed]
[30]. Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASHGastroenterology 2006 131(3):788-96.10.1053/j.gastro.2006.07.00716952548 [Google Scholar] [CrossRef] [PubMed]