Myeloid Sarcoma (MS) is an extra-medullary accumulation of the immature cells of granulocytic cell lineage. It has been reported commonly in the setting of acute myelogenous leukaemia, however its occurrence in Chronic Myeloid Leukaemia (CML), though reported as rare, is also common, although only isolated case reports are available. It is commonly misdiagnosed as non Hodgkin lymphoma. Occurrence of MS in a patient with CML defines the case as an extra medullary blast crisis, even without evidence of systemic disease. It is a tell-tale sign of poor prognosis. Here we describe a case of a 45-year-old female who presented with prolonged febrile illness with generalised lymphadenopathy who on extensive workup turned out to be MS as a first presentation of CML in blast crisis. We will review the intricacies of the case, discussing the diagnostic conundrum and treatment dilemma we faced and the systematic approach we followed to manage the patient.
Chemotherapy, Chronic myeloid leukaemia, Granulocytic sarcoma
Case Report
A 45-year-old lady presented to medicine emergency with chief complains of fever for three weeks and multiple swellings on her neck for the last two weeks (as shown in [Table/Fig-1a-c]). The swellings were insidious in onset and gradually progressive, non tender with no discharge. There were no complaints of cough, sputum production, bleeding manifestations, abdominal pain and distension, chest pain, bone pains and jaundice. There were no significant complaints of weight loss and loss of appetite. Past history was suggestive for pulmonary Koch’s 5 years back for which she completed 6 month course of anti-tubercular therapy. Rest of the personal, family and menstrual history was normal.
Multiple swellings over the neck (yellow arrows) representative of lymph node enlargement. a) lymph node enlargement in the left posterior triangle of the neck; b) lymph nodes in the sub mental area; c) lymph node enlargement in the right posterior triangle of the neck.
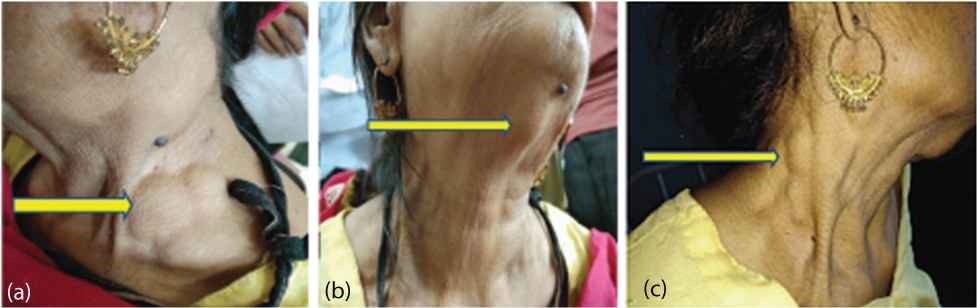
On examination, her vitals were stable and were afebrile. There was marked pallor and generalised cervical and bilateral axillary lymphadenopathy. The lymph nodes were firm in consistency, non mobile, non tender, around 1×2 cm in size approximately with no overlying redness or inflammation. Rest of the general physical examination was unremarkable. Her abdominal examination revealed hepatomegaly, around 1-2 cm below right costal margin with firm consistency and no tenderness along with splenomegaly, 2 cm below the costal margin. Rest of the respiratory, cardiovascular and CNS examination revealed no significant findings.
On further investigations, her Hb was 6.7 gm/dL with a TLC of 2400/mm3 and platelet count of 45000/mm3 suggestive of pancytopenia. Peripheral smear was suggestive of pancytopenia with RBC’s being normocytic and normochromic with a differential leucocyte count revealed 66% blasts and 16% of myelocytes and metamyelocytes, marked dyspoiesis in the myeloid lineage and marked basophilia suggestive of CML blast crisis. Serum LDH was raised to 1220 IU/mL and uric acid to 7.1 mg/dL. Rest of the serum biochemistry including KFT, LFT and ALP were within normal limits. ESR was elevated to 54 mm/hour. Sputum for AFB analysis was negative.
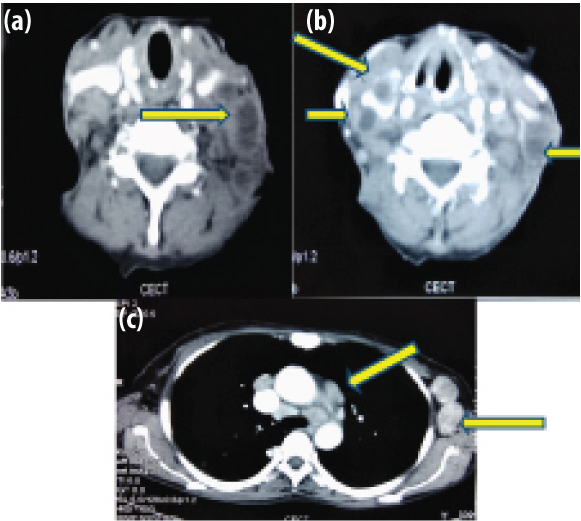
Ultrasound of the abdomen revealed hepatomegaly of 14.3 cm with a spleen of 11.3 cm. CECT of the neck, chest and abdomen [Table/Fig-2a-c] revealed multiple enlarged cervical lymph nodes in bilateral level II, III, IV, V and left Ia and Ib showing heterogenous post contrast enhancement with some of them showing conglomeration. There were multiple enlarged lymph nodes in the chest showing heterogeneous post contrast enhancement in bilateral axillary region with some showing conglomeration. There was lymphadenopathy in the prevascular, paratracheal, precarinal and subcarinal areas along with fibro bronchiectatic changes in the posterior segment of the right upper lobe suggestive of old infection.
CECT chest and neck is suggestive of multiple enlarged cervical lymph nodes (yellow arrows) in bilateral level II, III, IV, V and left Ia and Ib showing heterogeneous post contrast enhancement with some of them conglomeration. a,b) Multiple enlarged lymph nodes in the chest showing heterogeneous post contrast enhancement in bilateral axillary region with some showing conglomeration; c) lymphademapathy in the prevascular, paratracheal, precarinal and subcarinal areas.
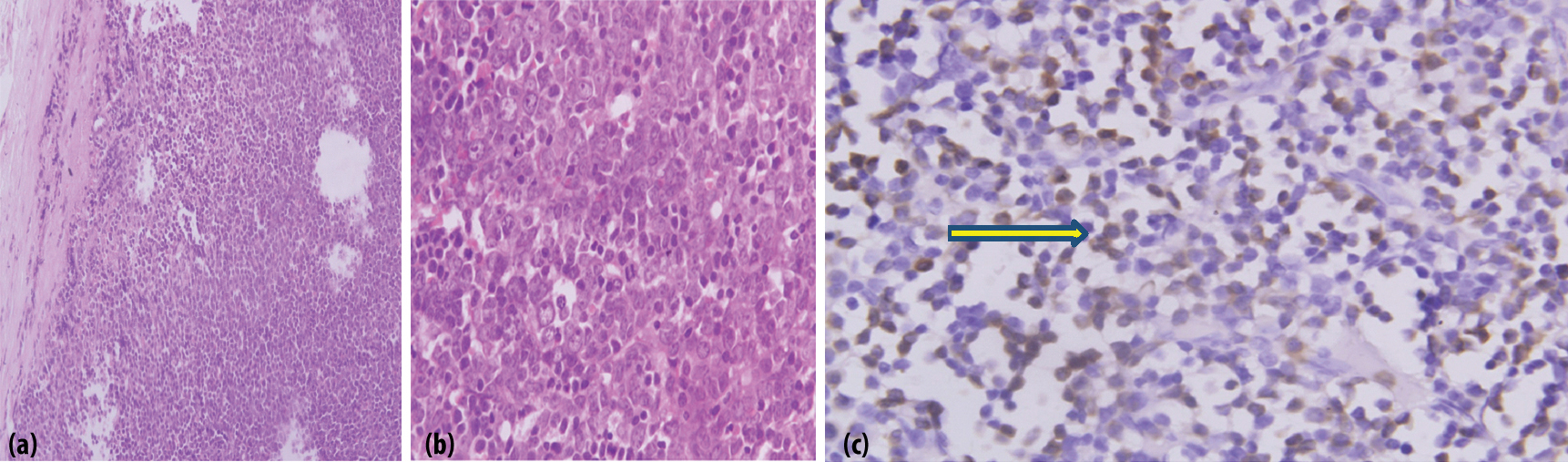
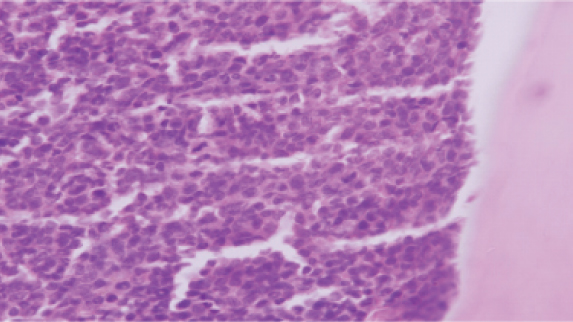
The patient was subjected to an FNAC of the cervical lymph nodes which was suggestive of small lymphocytes which were non cleaved and diffuse, most likely non Hodgkin lymphoma of the intermediate grade. The lymph node biopsy [Table/Fig-3a-c] was suggestive of effaced architecture of the lymph node with obliteration of the capsular sinus. There was infiltration of myelocytes and metamyelocytes with nucleated RBCs. There were cells having high N:C ratio and prominent nucleoli suggestive of blasts with strong positivity of Myeloperoxidase (MPO). All these features were suggestive of MS. Bone marrow aspirate with trephine biopsy, with peripheral smear [Table/Fig-4,5a,b] revealed CML with blast crisis. Flow cytometry of the blood sample was suggestive of myeloid blast crisis with myelomonocytic differentiation and aberrant CD 8 expression with positivity for CD13 (79.2%), CD13 and CD33 (35.8%), CD 64 (65.6%), cMPO (79%) with negative expression of CD10, CD19, CD79a, CD20, CD34, HJLADR, CD56, CD3, CD4 and CD7.
a) Lymph node biopsy with haematoxylin and eosin stain 10X and 40X magnification is suggestive of effaced architecture of the lymph node with obliteration the capsular sinus; b) the 40x magnification shows infiltration of myelocytes and metamyelocytes in the lymph node; c) There are cells having high N:C ratio and prominent nucleoli suggestive of blasts with strong positivity of myeloperoxidase (MPO) showing brown staining, as shown in immunohistochemistry panel of (yellow arrow), suggestive of myeloid sarcoma.
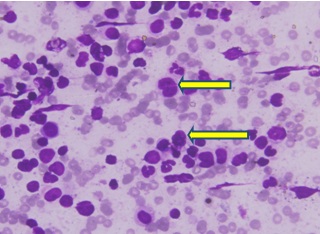
Peripheral smear is suggestive of 66% blasts (yellow arrows) and 16% of myelocytes and metamyelocytes. The blasts have moderate to abundant pale blue cytoplasm and marked dyspoiesis in the myeloid lineage and marked basophilia suggestive of CML blast crisis.
Bone marrow trephine biopsy on H&E stain, 10X magnification, is suggestive of bony trabeculae enclosing hypercellular marrow spaces with diffuse sheets of immature cells replacing normal marrow architecture. Blasts comprising of 66% of the marrow suggestive of CML in blast crisis.
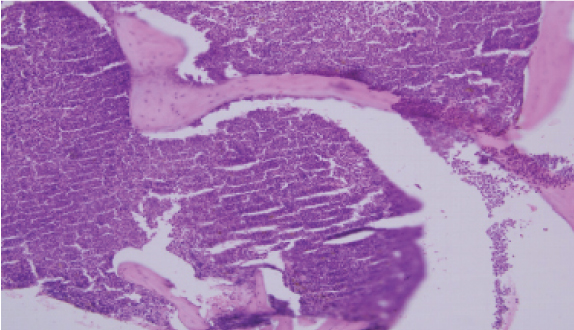
Depicts the paratrabecular location of the marrow. Normally the paratrabecular region is around 2-3 cell layer thick. The figure demonstrates the increase in thickness of more than 5-6 layers of immature cells suggestive of myeloproliferative neoplasm (H&E, 40X).
Based on the above clinical, radiological and histopathological findings, a final diagnosis of CML in blast crisis with a first presentation as lymph node MS was made and the patient was started immediately on Imatinib (800 mg) OD along with other supportive treatments (hydration, allopurinol and hydroxyurea with blood transfusions). Furthermore, later BCR-ABL mutation turned out to be positive. The patient responded dramatically to the treatment within one month and currently is on follow-up in present haematology OPD. There is marked decrease in the size of her lymph nodes and is in remission with no further complications.
Discussion
Chronic myeloid leukaemia is a clonal myeloproliferative disorder that is characterised by leukocytosis, granulocytic immaturity, basophilia, splenomegaly and a distinct genetic abnormality known as BCR-ABL fusion gene (Philadelphia chromosome) [1]. It is the most common adult leukaemia in India, accounting for 50-70% of all leukaemias with a triphasic clinical course: chronic phase (85%), accelerated phase (<10%) and blast crisis (2-3%) [2,3]. Blast phase is defined by the presence of peripheral blasts ≥20% of white blood cells or nucleated bone marrow cells, extramedullary blast proliferation or large foci or clusters of blasts on bone marrow biopsy [3].
MS is defined as a malignant extramedullary tumour of immature myeloid cell lineage [4]. As defined above, occurrence of MS in a patient with CML categorises the case as extramedullary blast crisis even without evidence of systemic disease. MS has been commonly reported in about 2-8% of the cases of AML and can occur after (50%), concurrent with (15-35%) or prior to the diagnosis of AML (25%), after Haematopoietic Stem Cell Transplantation (HSCT) (<1%) or rarely as the first sign of relapse after treatment of AML. MS has rarely been described in CML, Myelodysplastic Syndrome (MDS) and other myeloproliferative neoplasm [5].
MS is very commonly confused with NHL [6] because of morphologic similarities of the blasts to large cell lymphoma, the presence of lymphoglandular bodies, and the rarity of Auer rods and eosinophilic myelocytes. Diagnosis of MS requires high index of suspicion and gold standard diagnosis is histological examination with immunohistochemistry, especially showing negativity for CD30, CD3 and positivity for cMPO, which is a myeloid lineage marker [7].
A review of cases published on Pubmed using mesh terms like “lymph node MS in CML”, “CML MS” and “granulocytic sarcoma, CML” yielded isolated case reports of occurrence of MS in CML at unusual clinical sites with few of them citing lymph node involvement as well. However, in few of them, MS presents as a first sign leading to the diagnosis of CML. In a case report by Bangerter M et al., out of the 26 patients of granulocytic sarcoma who underwent FNAC, myeloid sarcoma preceeded the diagnosis of CML in only one patient [8]. Similarly, Paydas S et al., studied 32 cases of granulocytic sarcoma [9]. It was accompanied by AML in 13, ALL (My+) in one case, CML in 11 and MDS in two cases. Granulocytic sarcoma was diagnosed simultaneously with leukaemia in five and preceded the leukaemia in eight. Lymph node and soft tissue were the most common sites of involvement. Seven cases had first been diagnosed as NHL.
Few case reports have been published till date with MS as the first sign for the underlying diagnosis of CML, with majority in blast crisis is shown in [Table/Fig-6] [10-15] (Cases not published in English and age <10 years have been excluded).
Myeloid sarcoma as a heralding sign of CML [10-15].
| S. no. | Authors | Age/Sex | Clinical presentation | Diagnosis | Treatment given | Outcome |
|---|
| 1. | Chen X et al., [10] | 14 year/M | Right inguinal lymphadenopathy (3 months) | CML presenting as mixed phenotype (T/myeloid, bilineal) blast phase | AML protocol (mitoxantrone and Ara-C), dasatanib f/b BMT after 5 months | Survived, in remission |
| 2. | Kumar V et al., [11] | 25 year/F | localised swellings over posterolateral aspects of both thighs (2 months) | CML (chronic phase) presenting as MS | hydroxyurea 1000 mg twice daily | Survived |
| 3. | Torres F et al., [12] | 18 year/F (clinical course as 4 presentations) | a. iliac granulocytic sarcoma.b. relapse as CML (blast crisis)c. second relapseCML-BC, with CNS involvementd. progression of iliac tumour | a. diagnosed as CML (CP)b. CML (blast crisis)c. CML (blast crisis) | a. radiotherapy and 400 mg/day of IMb. 800 mg/day of IMc. Imatinib (800 mg/day) | Died after the fourth presentation |
| 4. | Nagarajarao HS et al., [13] | 53 year/M | multiple skin nodules on the right side of his chest, right arm, left thigh and left shin | CML (CP) with myeloid sarcoma | Imatinib | Survived |
| 5. | Ai D et al., [14] | 23 year/F | Right inguinal lymphademapathy (7 months) | CML (Blast crisis) presenting as MS (e1a2 BCR-ABL1 transcript) | Idarubicin, cytarabine, vincristine, dexamethasone and imatinib (600 mg/day) followed by BMT | Survived |
| 6. | Levy RA et al., [15] | 35 year/M | right posterior shoulder pain | CML (Blast crisis) presenting as MS | Dasatinib (140 mg OD) f/b BMT | Survived |
In present case too, an initial diagnosis of NHL was made on FNAC which was later confirmed to be MS on lymph node biopsy and immunohistochemistry. The use of special immunohistochemical stains is mandatory, because this tumor is often misdiagnosed as malignant lymphoma. MS are regarded as a heralding sign of a systemic relapse. The extensive distribution of the lymphadenopathy as MS as a first sign of an underlying CML blast crisis makes it an unusual presentation, as highlighted in present case report. The early diagnosis and prompt institution of chemotherapy leads to dramatic improvement and longer survival.
Conclusion
This case report highlights the importance that CML presenting as MS is not a rare entity as described in literature. Prompt diagnosis with the help of biopsy with immunohistochemistry of the involved organ is of utmost importance in favourable outcome of the patient. In present case, only with Imatinib (TKI), the patient had a dramatic response in terms of clinical improvement in the size of the swellings and haematological as well as radiological parameters and is currently in remission on regular follow-up with no relapse till date. This case report also negates the fact that MS can have a good prognosis if diagnosed and treated well on time.
[1]. Chauffaille MD, Myeloproliferative neoplasms: a review of diagnostic criteria and clinical aspectsRev. Bras. Hematol. Hemoter 2010 32(4):308-16.10.1590/S1516-84842010005000091 [Google Scholar] [CrossRef]
[2]. Bansal S, Prabhash K, Parikh P, Chronic myeloid leukaemia data from IndiaIndian J Med Paediatr Oncol 2013 34(3):154-58.10.4103/0971-5851.12371124516297 [Google Scholar] [CrossRef] [PubMed]
[3]. Samborska M, Derwich K, Skalska-Sadowska J, Kurzawa P, Wachowiak J, Myeloid sarcoma in children–diagnostic and therapeutic difficultiesContemp Oncol 2016 20(6):444-48.10.5114/wo.2016.6560228239280 [Google Scholar] [CrossRef] [PubMed]
[4]. Yu T, Xu G, Xu X, Yang J, Ding L, Myeloid sarcoma derived from the gastrointestinal tract: A case report and review of the literatureOncol Lett 2016 11(6):4155-59.10.3892/ol.2016.451727313759 [Google Scholar] [CrossRef] [PubMed]
[5]. Avni B, Koren-Michowitz M, Myeloid sarcoma: current approach and therapeutic optionsTher Adv Haematol 2011 2(5):309-16.10.1177/204062071141077423556098 [Google Scholar] [CrossRef] [PubMed]
[6]. Jain MF, Khandekar SL, Mahadani JW, Raut WK, Report of a case of primary breast lymphoma highlighting the importance of fine needle aspiration cytology as an initial diagnostic toolJ Cytol 2015 32(2):127-29.10.4103/0970-9371.16057026229252 [Google Scholar] [CrossRef] [PubMed]
[7]. Kremer M, Quintanilla-Martínez L, Nährig J, von Schilling C, Fend F, Immunohistochemistry in bone marrow pathology: a useful adjunct for morphologic diagnosisVirchows Arch 2005 447(6):920-37.10.1007/s00428-005-0070-816231177 [Google Scholar] [CrossRef] [PubMed]
[8]. Bangerter M, Hildebrand A, Waidmann O, Griesshammer M, Diagnosis of granulocytic sarcoma by fine-needle aspiration cytologyActa Haematol 2000 103(2):102-08.10.1159/00004102810838454 [Google Scholar] [CrossRef] [PubMed]
[9]. Paydas S, Zorludemir S, Ergin M, Granulocytic sarcoma: 32 cases and review of the literatureLeuk Lymphoma 2006 47(12):2527-41.10.1080/1042819060096719617169797 [Google Scholar] [CrossRef] [PubMed]
[10]. Chen X, Rutledge JC, Wu D, Fang M, Opheim KE, Xu M, Chronic myelogenous leukaemia presenting in blast phase with nodal, bilineal myeloid sarcoma and T-lymphoblastic lymphoma in a childPediatr Dev Pathol 2013 16(2):91-96.10.2350/12-07-1230-CR.123171293 [Google Scholar] [CrossRef] [PubMed]
[11]. Kumar V, Jain N, Chaudhary SC, Mishra S, Multiple skin chloromas: a rare presentation of chronic myelogenous leukaemia in chronic stable phaseBMJ Case Rep 2013 2013:bcr201300862610.1136/bcr-2013-00862623592814 [Google Scholar] [CrossRef] [PubMed]
[12]. Torres F, Ivanova-Dragoeva A, Pereira M, Veiga J, Rodrigues AS, Sousa AB, An e6a2 BCR-ABL fusion transcript in a CML patient having an iliac chloroma at initial presentationLeuk Lymphoma 2007 48(5):1034-37.10.1080/1042819070121640217487751 [Google Scholar] [CrossRef] [PubMed]
[13]. Nagarajarao HS, Akhtar I, Heard K, Baliga M, Unusual presentation of chronic myelogenous leukaemia as multiple skin chloromasActa Cytol 2009 53(2):235-38.10.1159/00032513319365984 [Google Scholar] [CrossRef] [PubMed]
[14]. Ai D, Liu W, Lu G, Patel KP, Chen Z, Extramedullary blast crisis as initial presentation in chronic myeloid leukaemia with the e1a2 BCR-ABL1 transcript: A case reportMol Clin Oncol 2015 3(6):1319-22.10.3892/mco.2015.64126807241 [Google Scholar] [CrossRef] [PubMed]
[15]. Levy RA, Mardones MA, Burch MM, Krause JR, Myeloid sarcoma as the presenting symptom of chronic myelogenous leukaemia blast crisisProc (Bayl Univ Med Cent) 2014 27(3):246-49.10.1080/08998280.2014.1192912724982578 [Google Scholar] [CrossRef] [PubMed]