Introduction
Rheumatoid Arthritis (RA) is the most common inflammatory arthritis which primarily affects the synovial lining of joints. Tendon sheath is also composed of synovial lining and tendon involvement in RA is generally in the form of tenosynovitis. Modern imaging techniques like Ultrasonography (USG) and Magnetic Resonance Imaging (MRI) target mainly soft tissue pathological changes in RA, and have lead to the early diagnosis and prognostication of disease, which in turn has guided rheumatologist to start biologic therapy in the early stage of disease and thereby prevent complications.
Aim
To evaluate flexor tenosynovitis in untreated early RA using USG and to compare its findings with that of MRI along with studying distribution of flexor tenosynovitis using both USG and MRI across hand.
Materials and Methods
The present hospital based descriptive study was conducted in the Department of Radiodiagnosis of Subharti Medical College and hospital from January to December 2016. A total of 40 patients of RA underwent high frequency USG and MRI of 2nd to 5th Flexor Tendon Sheaths (FTS) of both hands whereas 25 healthy controls underwent only high frequency USG. Normal anatomy and inflammatory changes in the FTS were recorded on both the modalities by two radiologists specialised in doing musculoskeletal imaging. No patient had received prior steroid or Disease-Modifying Antirheumatic Drug (DMARD). Diagnostic accuracy of USG was calculated using MRI as gold standard using agreement statistics. Statistical analysis was done using SPSS version 12.0.
Results
Flexor tenosynovitis was found in 102 (31.8%) of 320 and FTS in 22 (55%) of 40 patients on ultrasound compared with 210 (65.6%) of 320 with Flexor tensosynovitis on MRI. FTS were completely normal in control subjects on ultrasound. Considering MRI as the gold standard, the sensitivity, specificity, negative and positive predictive values for ultrasound were 0.52, 0.83, 0.69, and 0.70, respectively, for detecting flexor tenosynovitis. The most frequently involved FTS on both the modalities were the second and third.
Conclusion
Both ultrasound and MRI can be used for detection of flexor tenosynovitis in patients with early untreated RA. MRI is more sensitive for detecting flexor tenosynovitis for obvious reasons. A negative ultrasound scan does not exclude inflammation and an MRI should be considered. In developing countries like India, where MRI is limited in availability, ultrasound can become the imaging modality of choice specially to evaluate soft tissue changes in early RA.
Cross sectional imaging, Finger tendons, Gray scale imaging, Inflammatory arthritis, Power doppler imaging
Introduction
Rheumatoid arthritis is the most common inflammatory arthritis and chronic autoimmune inflammatory disorder which primarily affects the synovial lining of joints and affects approximately 1% of the world’s population. Conventional radiography has been the imaging modality of choice in RA, however it can provide only indirect information on synovial inflammation and it is insensitive to early joint damage. With the advent of new therapeutic agents like anti tumour necrosis factor agents, which are quite potent and expensive drugs, has created the need for early diagnosis and measurement of disease activity. The therapeutic window described in RA is the first three months of disease duration. If aggressive treatment is started early within the therapeutic window, it is possible to suppress the inflammatory reaction, otherwise the disease can take a more chronic and aggressive course [1,2].
Tendon sheath is also composed of synovial lining and tendon involvement in RA is generally in the form of tenosynovitis. Tenosynovitis predominates over joint synovitis in some patients and can be the only pathological finding in some patients with RA. Tenosynovitis is postulated to be the earliest manifestation of RA and is usually bilateral. In some situations, inflammation is also seen in the tendons secondary to tenosynovitis which can lead to tendon thinning and rupture. The USG and MRI being highly sensitive modalities to detect soft tissue pathological changes in RA can help in making early diagnosis. These modalities also help in prognostication of disease and guide rheumatologist to start modern treatment as early as possible and thereby prevent complications [1,2].
Composite scores used in RA for both clinical purpose and trials depend on counting swollen and tender joints and include Disease Activity Score for 28 joints (DAS28), Simplified Disease Activity Index (SDAI), and Clinical Disease Activity Index (CDAI) [3,4].
The RA is the major disease which is most extensively studied using high frequency USG. Through its inborn ability to detect synovial proliferation and increased vascularity, USG has gained superiority in detecting synovitis as compared to the clinical examination alone. Tendon sheaths can be accessed through USG along their course up to the insertion. It can be assessed using gray scale as well as doppler. Ultrasonographic evaluation has proven to add more value over clinical examination in assessing the disease activity in RA. USG has capability to diagnose tenosynovitis separately from synovitis and determine the exact cause of swelling [5,6]. USG has been regarded as gold standard imaging method for evaluation of superficial tendons in patients with RA [7]. Outcome Measures in Rheumatology in Clinical Trials (OMERACT) Ultrasound Task force group has regarded tenosynovitis and tendonitis in RA as one of the main direction for current and future research [8].
On ultrasound evaluation, normal flexor tendon appears hyperechoic, uniform in thickness and echogenicity with fibrillar echopattern [Table/Fig-1]. Synovial sheath of the tendon appears as thin hypoechoic halo surrounding the tendon seen in longitudinal and transverse planes. Tenosynovitis was observed as thin or thick layer of synovial membrane with or without associated fluid and increased vascularity which can be detected as foci of increased doppler activity on color or power doppler [Table/Fig-2a,b]. Earliest marker of tendon damage is loss of fibrillar echopattern which is unique quality of normal tendon. Partial or full thickness tendon tears are defined as interruption of tendon fibers with or without hypoechoic material filling the defect [9].
Ultrasonography of normal flexor tendon appearing hyperechoic, uniform in thickness and echogenicity with fibrillar echopattern.
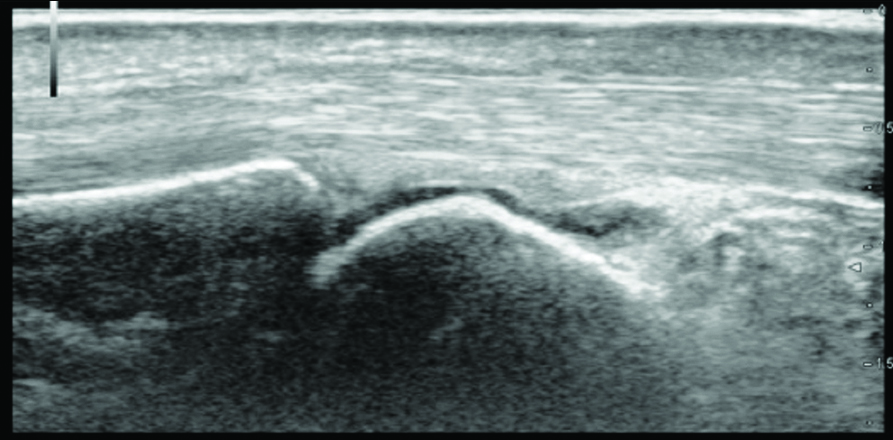
Flexor tenosynovitis in one of the patients is observed as thick layer of synovial membrane around the tendon on high frequency gray scale ultrasonography.
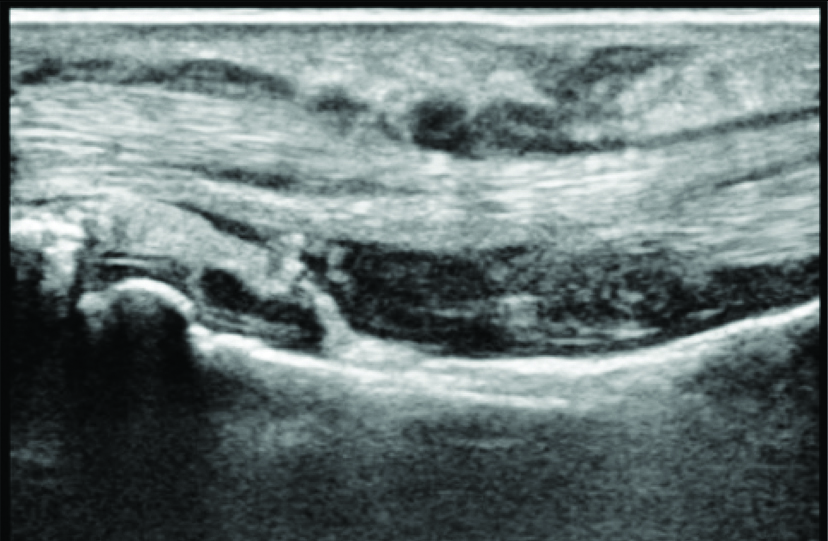
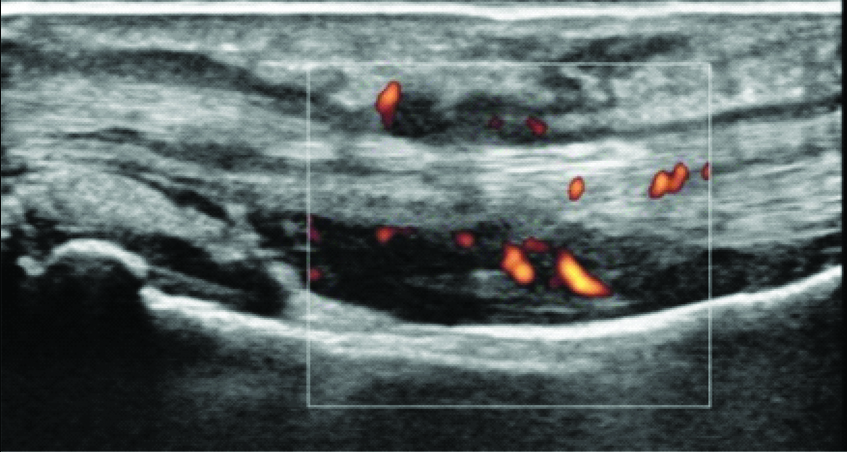
Ultrasonography showing tenosynovitis as thick layer of synovial membrane around flexor tendon with increased vascularity detected as foci of increased doppler activity.
Not only early diagnosis but measurement of RA disease activity has become equally important due to new application of Disease Modifying Anti-Rheumatic Drugs (DMARD) in RA treatment. Clinical remission can be defined as complete absence of synovitis associated signs and symptoms and different composite scores have been developed to measure the same. However, counting tender and swollen joints depends on clinician’s experience and so insensitivity exists in clinical assessment of true arthritic activity. Deformed joint with fibrous tissue only can’t be differentiated from true swollen joint on the basis of clinical assessment alone. Patient’s different pain tolerability can also lead to inaccurate assessment as can false negative inflammatory markers. Clinical parameters do not reflect a true absence of synovitis and thus remission seen on imaging (USG/MRI) as a complete absence of synovial inflammation has emerged as an alternative criteria to diagnose remission in RA. Therefore, power doppler remission can be a new therapeutic target in the treatment of RA [10].
Doppler signals act as reflection of joint inflammation. Many studies have found that doppler signals detected in the synovial joint are correlated with enhancing synovial membrane seen on MRI or hypervascularity detected on histopathology [11,12]. Power doppler is less incidence angle dependent, does not produce aliasing artifact inherently and more sensitive to microvascular flow and so in rheumatological ultrasound examination, power doppler is preferred over color doppler [13].
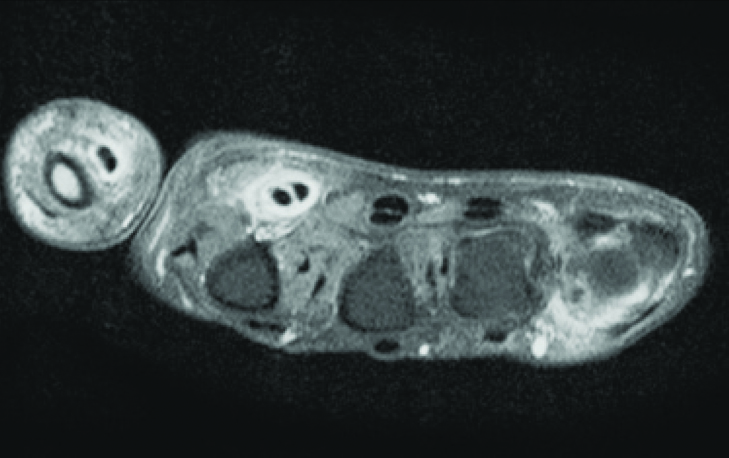
The MRI on the other hand, has an edge over ultrasound due to its excellent soft tissue resolution and multi-planar capability. Post gadolinium enhanced MRI can visualise the thickened and enhancing synovium which represents the synovial inflammation and therefore, it can be used as unique marker of synovitis [Table/Fig-2c]. According to European society of musculoskeletal radiology arthritis subcommittee and European league against rheumatism recommendations, MRI is presently considered as the best, non invasive, observer independent imaging modality for rheumatological evaluation [14].
Post gadolinium enhanced MRI axial section showing thickened and enhancing synovium which represents synovial inflammation around flexor tendon of first and second digits.
Both USG and MRI can detect synovitis sensitively and play an important role in the diagnosis and management of RA. Both the modalities play complementary role in most of the rheumatological imaging investigations. The present study was conducted with an aim to evaluate flexor tenosynovitis in untreated early RA using USG and to compare its findings with that of MRI along with studying distribution of flexor tenosynovitis using both USG and MRI across hand.
Materials and Methods
The present hospital based descriptive study was conducted in the Department of Radiodiagnosis of Subharti Medical College and hospital from January to December 2016. The study protocol was approved by Institutional Research Ethical Committee and informed consent was obtained from all the patients. Patients with early RA (disease duration less than six months) as defined by American College of Rheumatology (ACR) criteria 2010 were consecutively recruited from rheumatology clinic at present institute [15]. None of the patient had received prior treatment with DMARD or steroids. Sample size was calculated by the formula “n≥4xSD2/m2”. Where n is sample size, m is margin of error and SD is standard deviation.
A total of 40 patients with symptomatic involvement of metacarpophalangeal joints were selected by recruiter along with 25 age and gender matched healthy controls. Uncooperative patients and patients in whom MRI was contraindicated due to implanted medical devices were excluded from the study. Patients already taking treatment and having deformities were also excluded. Variables like age, gender, disease duration, Rheumatoid Factor (RF), C-Reactive Protein (CRP), Anti Citrullinated Peptide Antibody (ACPA) and Erythrocyte Sedimentation Rate (ESR) were recorded. All the investigations and MRI were performed within two days of ultrasound examination.
Ultrasound evaluation: Patients were comfortably seated with hands kept on pillow. High end ultrasound system with power doppler was used for examination. Frequency range was kept 7-14 MHz and proper optimisation of the gray scale settings was performed. Generous amount of ultrasound gel was used to avoid manually compressing the tendon sheaths with ultrasound transducer. Radiologist having experience in musculoskeletal ultrasonography examined 2nd to 5th FTS of both hands of the patients as well as controls and was unaware of the patient’s symptoms. FTS were examined on gray scale from the level of wrist up to their insertion in both longitudinal and transverse planes followed by power doppler to assess the vascularity in the area of abnormality. The presence or absence of flexor tenosynovitis was documented.
MRI evaluation: MRI of both hands of the patients was performed using a commercially available 1.5 Tesla scanner with image acquisition using surface coils. MRI of controls was not performed. Patients were placed in prone position with hands placed above head over the sand bags. Patients were instructed to avoid any movement during the examination. A cannula was placed in right antecubital vein before sending the patients into the scanner. Following sequences were obtained: T1 weighted spin echo in coronal and axial planes, T2 weighted turbo spin echo fat suppressed in coronal and axial planes, and T1 weighted fat suppressed post gadolinium diethylenetriaminepentaacteic acid in axial and coronal planes.
The MRI images were analysed by another radiologist experienced in musculoskeletal imaging, who was blinded about the patient’s clinical and ultrasound findings. Flexor tenosynovitis was identified as thickened sheaths with increased enhancement on post contrast images as compared to the pre-contrast ones and it was recorded as present or absent.
Statistical Analysis
Considering MRI as gold standard, diagnostic accuracy of Ultrasonography was calculated as sensitivity, specificity, Positive Predictive Value (PPV) and Negative Predictive Value (NPV). The statistical analysis was done by using SPSS 12.0 (SPSS, Chicago, IL).
Results
Characteristics of 40 patients are represented in [Table/Fig-3]. A total of 320 FTS were examined using both USG and MRI and subsequent comparison was done using MRI as gold standard. Flexor Tenosynovitis was found in 102 (31.8%) of 320 FTS in 22 (55%) of 40 patients on ultrasound compared with 210 (65.6%) of 320 FTS in 32 (80%) of 40 patients on MRI. FTS were completely normal in control subjects on ultrasound. Considering MRI as the gold standard, the sensitivity, specificity, negative and positive predictive values for ultrasound are tabulated in [Table/Fig-4], for detecting flexor Tenosynovitis. Most frequently involved FTS using both the modalities were the second and third. The distribution of FTS involved across hands using USG and MRI is represented in [Table/Fig-5].
Characteristics of the patients.
| S No. | Characteristics of the patients | Mean±standard deviation |
|---|
| 1 | Mean age of patients (years) | 39.333±20.92 |
| 2 | Mean duration of disease (months) | 3.933±2.514 |
| 3 | RF titre (IU/mL) | 37.85±17.54 |
| 4 | ESR (mm/hr) | 36.73±30.95 |
| 5 | CRP level (mg/L) | 8.79±10.09 |
| 6 | ACPA (EU) | 45.12±24.32 |
RF: Rheumatoid factor; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein; ACPA: Anti-citrullinated protein antibodies; IU: International unit; mL: Milliliter; mm: Millimeter; hr: Hour; L: Liter: mg: Milligram
Table showing sensitivity, specificity, positive predictive value and negative predictive value of ultrasonography as compared to MRI for detection of flexor tenosynovitis.
| Sensitivity | Specificity | Positive predictive value | Negative predictive value |
|---|
| Flexor tenosynovitis | 0.52 | 0.83 | 0.69 | 0.70 |
Table showing distribution of flexor tenosynovitis across hands using USG and MRI.
| FTS 1 | FTS 2 | FTS 3 | FTS 4 | Total (320 FTS) |
|---|
| USG | 19 | 24 | 37 | 22 | 102 (31.8%) |
| MRI | 30 | 55 | 76 | 49 | 210 (65.6%) |
USG: Ultrasonography; MRI: Magnetic resonance imaging; FTS: Flexor tendon sheath
Discussion
In the present study, flexor tenosynovitis was found in quite large no of FTS examined on both USG and MRI. Compared to MRI, USG has moderate sensitivity but high specificity, PPV and NPV for detecting flexor tenosynovitis.
Hmamouchi I et al., in their study compared clinical examination with USG in detecting clinical and subclinical synovitis and tenosynovitis in early RA. They concluded that clinical examination has high specificity and positive predictive value and so act as a valuable tool for detecting flexor tenosynovitis but on the other hand a negative clinical examination cannot exclude inflammation and USG is advisable [16]. Naredo E et al., compared the clinical evaluation of inflammatory activity in patients with RA with gray scale and power doppler USG and found that USG showed significantly more number of joints with effusion and synovitis than clinical examination (p<0.05) [6].
Walther M et al., examined the significance of power doppler USG in the diagnosis of synovial hypertrophy of the knee joint by comparing the Ultrasonographic findings with histopathologic findings of synovial membrane vascularity. They found that power doppler USG proved to be a reliable diagnostic method for qualitative grading of the vascularity of the synovial tissue [13].
Wakefield RJ et al., determined the frequency and distribution of finger tenosynovitis in non treated early RA patients with USG and MRI. In their study, 28% of FTS and 14% of Extensor Tendon Sheaths (ETS) showed sign of inflammation on USG whereas the data were 64% and 40% respectively on MRI. Control subjects in their study too did not show imaging signs of tenosynovitis [17]. McQueen F et al., studied a cohort of patients with RA over six years for occurrence, pattern and progression of tendinopathy and found that MRI can be used to quantify tendinopathy at wrist joint and those with high scores in early disease were more predictive of tendon rupture in their disease course [18].
Eshed I et al., studied 99 patients with unspecified arthritis or suspected RA having no findings on conventional radiographs with MRI as standalone tool and found that Flexor tenosynovitis diagnosed by MRI of the hand is a strong predictor of early RA [19]. van Steenbergen HW et al., in their study found that Subclinical inflammation was present in 44% of patients with clinically suspected arthritis as measured by MRI and 35% of them progressed to clinically evident inflammation in four months [20].
In patients undergoing treatment, it is important for the clinician to detect remission. It is well known fact that in many patients with RA who are in stage of clinical remission continues to have persistent inflammation at microscopic level. USG and MRI can sensitively detect synovitis and bone marrow oedema which generally persist even in phase of clinical response. However after successful treatment, MRI and USG will show fibrotic pannus which appears relatively hypointense on MRI after post gadolinium contrast imaging sequences and shows decreased doppler activity on power doppler ultrasonography. On T2-weighted MRI sequence, fibrotic pannus will show intermediate to low signal intensity as compare to high signal intensity of acutely inflamed synovium [10].
Brown AK et al., first suggested the importance of imaging remission in RA [21]. Peluso G et al., determined different chances of relapse in early and long standing RA on the basis of clinical and imaging criteria. They found that power doppler remission occurred in more fraction of patients (43.7%) in early RA than in long standing RA (17.4%) in phase of clinical remission [22]. These studies support the fact that power doppler remission is more clinically significant than clinical remission and presence of doppler activity in the synovium at any given situation suggests inflammation.
Limitation
The present study had some drawbacks as patients were not followed on treatment to analyse imaging remission. Future studies with more number of patients and with follow-up on treatment are required to assess these data. Further work is recommended to standardise parameters of image acquisition and criteria for characterisation of peritendinous inflammation on both USG and MRI.
Conclusion
In conclusion, the present study compared USG and MRI for detection of flexor tenosynovitis in hands of patients with early untreated RA. Flexor tenosynovitis was found to be common using both the modalities highlighting the importance of imaging over clinical evaluation. Although, MRI seems to be more sensitive, USG had some definitive advantages over MRI like ease of performance, dynamicity, repeatability etc. A negative USG scan does not exclude inflammation and MRI is recommended.
RF: Rheumatoid factor; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein; ACPA: Anti-citrullinated protein antibodies; IU: International unit; mL: Milliliter; mm: Millimeter; hr: Hour; L: Liter: mg: Milligram
USG: Ultrasonography; MRI: Magnetic resonance imaging; FTS: Flexor tendon sheath
[1]. Narváez JA, Narváez J, De Lama E, De Albert M, MR imaging of early rheumatoid arthritisRadiographics 2010 30(1):143-63.discussion 163-6510.1148/rg.30109508920083591 [Google Scholar] [CrossRef] [PubMed]
[2]. Boutry N, Morel M, Flipo RM, Demondion X, Cotton A, Early rheumatoid arthritis: a review of MRI and sonographic findingsAJR Am J Roentgenol 2007 189:1502-09.10.2214/AJR.07.254818029892 [Google Scholar] [CrossRef] [PubMed]
[3]. Prevoo ML, van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL, Modified disease activity scores that include twenty-eight joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritisArthritis Rheum 1995 38:44-48.10.1002/art.17803801077818570 [Google Scholar] [CrossRef] [PubMed]
[4]. Aletaha D, Smolen J, The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritisClin Exp Rheumatol 2005 23:S100-08. [Google Scholar]
[5]. Alcalde M, D’Agostino MA, Bruyn GA, Möller I, Iagnocco A, Wakefield RJ, A systematic literature review of US definitions, scoring systems and validity according to the OMERACT filter for tendon lesion in RA and other inflammatory joint diseasesRheumatology (Oxford) 2012 51(7):1246-60.10.1093/rheumatology/kes01822378717 [Google Scholar] [CrossRef] [PubMed]
[6]. Naredo E, Bonilla G, Gamero F, Uson J, Carmona L, Laffon A, Assessment of inflammatory activity in rheumatoid arthritis: a comparative study of clinical evaluation with grey scale and power Doppler ultrasonographyAnn Rheum Dis 2005 64(3):375-81.10.1136/ard.2004.02392915708891 [Google Scholar] [CrossRef] [PubMed]
[7]. Grassi W, Filippucci E, Farina A, Cervini C, Sonographic imaging of tendonsArthritis Rheum 2000 43(5):969-76.10.1002/1529-0131(200005)43:5<969::AID-ANR2>3.0.CO;2-4 [Google Scholar] [CrossRef]
[8]. Naredo E, Wakefield RJ, Iagnocco A, Terslev L, Filippucci E, Gandjbakhch F, The OMERACT ultrasound task force status and perspectivesJ Rheumatol 2011 38(9):2063-67.10.3899/jrheum.11042521885518 [Google Scholar] [CrossRef] [PubMed]
[9]. Naredo E, D’Agostino MA, Wakefield RJ, Moller I, Balint PV, Filippucci E, Reliability of a consensus-based ultrasound score for tenosynovitis in rheumatoid arthritisAnn Rheum Dis 2013 72(8):1328-34.10.1136/annrheumdis-2012-20209222984169 [Google Scholar] [CrossRef] [PubMed]
[10]. McQueen FM, The MRI view of the synovitis and tenosynovitis in inflammatory arthritis: implications for diagnosis and managementAnn NY Acad Sci 2009 1154:21-34.10.1111/j.1749-6632.2009.04382.x19250228 [Google Scholar] [CrossRef] [PubMed]
[11]. Farrant JM, O’Connor PJ, Grainger AJ, Advanced imaging in rheumatoid arthritis Part 1: synovitisSkeletal Radiol 2007 36(4):269-79.10.1007/s00256-006-0219-917139505 [Google Scholar] [CrossRef] [PubMed]
[12]. Bhasin S, Cheung PP, The role of power doppler ultrasonography as disease activity marker in rheumatoid arthritisDis Markers 2015 2015:32590910.1155/2015/32590926063952 [Google Scholar] [CrossRef] [PubMed]
[13]. Walther M, Harms H, Krenn V, Radke S, Faehndrich TP, Gohlke F, Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritisArthritis Rheum 2001 44(2):331-38.10.1002/1529-0131(200102)44:2<331::AID-ANR50>3.0.CO;2-0 [Google Scholar] [CrossRef]
[14]. Sudoł-Szopińska I, Jurik AG, Eshed I, Lennart J, Grainger A, Østergaard M, Recommendations of the ESSR arthritis subcommittee for the use of magnetic resonance imaging in musculoskeletal rheumatic diseasesSemin Musculoskelet Radiol 2015 19(4):396-411.10.1055/s-0035-156469626583367 [Google Scholar] [CrossRef] [PubMed]
[15]. Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, American College of Rheumatology guideline for the treatment of rheumatoid arthritisArthritis Rheumatol 2016 68(1):1-26.10.1002/art.3948026545940 [Google Scholar] [CrossRef] [PubMed]
[16]. Hmamouchi I, Bahiri R, Srifi N, Aktaou S, Abouqal R, Hajjaj-Hassouni N, A comparison of ultrasound and clinical examination in the detection of flexor tenosynovitis in early arthritisBMC Musculoskelet Disord 2011 12:9110.1186/1471-2474-12-9121549008 [Google Scholar] [CrossRef] [PubMed]
[17]. Wakefield RJ, O’Connor PJ, Conaghan PG, McGonagle D, Hensor EM, Gibbon WW, Finger tendon disease in untreated early rheumatoid arthritis: a comparison of ultrasound and magnetic resonance imagingArthritis Rheum 2007 57(7):1158-64.10.1002/art.2301617907233 [Google Scholar] [CrossRef] [PubMed]
[18]. McQueen F, Beckley V, Crabbe J, Robinson E, Yeoman S, Stewart N, Magnetic resonance imaging evidence of tendinopathy in early rheumatoid arthritis predicts tendon rupture at six yearsArthritis Rheum 2005 52(3):744-51.10.1002/art.2094715751075 [Google Scholar] [CrossRef] [PubMed]
[19]. Eshed I, Feist E, Althoff CE, Hamm B, Konen E, Burmester GR, Tenosynovitis of the flexor tendons of the hand detected by MRI: an early indicator of rheumatoid arthritisRheumatology (Oxford) 2009 48(8):887-91.10.1093/rheumatology/kep13619474128 [Google Scholar] [CrossRef] [PubMed]
[20]. van Steenbergen HW, van Nies JA, Huizinga TW, Bloem JL, Reijnierse M, van der Helm-van Mil AH, Characterising arthralgia in the preclinical phase of rheumatoid arthritis using MRIAnn Rheum Dis 2015 74(6):1225-32.10.1136/annrheumdis-2014-20552224718962 [Google Scholar] [CrossRef] [PubMed]
[21]. Brown AK, Quinn MA, Karim Z, Conaghan PG, Peterfy CG, Hensor E, Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progressionArthritis Rheum 2006 54(12):3761-73.10.1002/art.2219017133543 [Google Scholar] [CrossRef] [PubMed]
[22]. Peluso G, Michelutti A, Bosello S, Gremese E, Tolusso B, Ferraccioli G, Clinical and ultrasonographic remission determines different chances of relapse in early and long standing rheumatoid arthritisAnn Rheum Dis 2011 70(1):172-75.10.1136/ard.2010.12992421097799 [Google Scholar] [CrossRef] [PubMed]