Primary Large Cell (Atypical) Ewing Sarcoma/Primitive Neuroectodermal Tumour of Kidney
Kiran Agarwal1, Reema Bhushan2, Partap Singh Yadav3
1 Director Professor, Department of Pathology, Lady Hardinge Medical College, Delhi, India.
2 Senior Resident, Department of Pathology, Lady Hardinge Medical College, Delhi, India.
3 Associate Professor, Department of Paediatric Surgery, Lady Hardinge Medical College, Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Reema Bhushan, 1113, Sector-4 RK Puram, Delhi-110022, India.
E-mail: drreems25@gmail.com
Primary Ewing Sarcoma/Primitive Neuroectodermal Tumour (ES/PNET) of the kidney is a rare entity. Here we describe a case of renal ES/PNET in 10-year-old girl who complained of abdominal pain and lump for a month. Contrast Enhanced Computed Tomography (CECT) revealed a huge heterogeneous solid cystic mass in the right kidney. We received a distorted specimen of kidney with rupture at upper pole and lateral wall. Sections from the renal mass showed an unencapsulated tumour separated into multiple nodules by thin fibrous septa with areas of extensive haemorrhage and necrosis. The tumour was composed of large round cells with moderate amount of clear to eosinophilic cytoplasm, round to irregular nucleus showing moderate to marked nuclear pleomorphism, coarse granular chromatin and prominent nucleolus. Mitotic figure were 3-4/10 high power fields. Differential diagnosis of large cell lymphoma, malignant melanoma, rhabdoid tumour, rhabdomyosarcoma and atypical ES were considered. Immunohistochemistry revealed diffuse strong positivity for CD99 with focal positivity for vimentin, Neuron Specific Enolase (NSE) and Cytokeratins (CK). Periodic Acid Schiff (PAS) stain showed intra cytoplasmic glycogen in few cells. Hence, the final diagnosis of large cell (atypical) ES/PNET was given. This case report highlights rare occurrence of primary renal large cell (atypical) ES/PNET.
Large round cells, Nuclear pleomorphism, Tumour
Case Report
A 10-year-old female complained of on and off dull abdominal pain along with slowly growing abdominal lump for a month. Physical examination revealed a large non tender palpable mass involving right hypochondrium, right lumbar and umbilical region. Provisional clinical diagnosis of renal cell carcinoma and Wilms tumour were considered. Laboratory investigations including haemogram and serum biochemistry were within normal limits. Patient did not have any complaint of haematuria. CECT abdomen revealed a well defined heterogeneous solid cystic mass replacing the right kidney displacing the surrounding structures, suggesting a neoplastic lesion [Table/Fig-1]. Left kidney was normal. Ultrasound guided fine needle aspiration from the mass done twice which revealed only blood. Ascitic fluid cytology showed reactive mesothelial cells and macrophages. No malignant cell was seen. Patient underwent right radical nephrectomy. A distorted specimen of right kidney with few soft tissue bits was received measuring 14×8×6 cm, weighing 600 grams with rupture at upper pole and lateral wall. Most of the renal parenchyma except the area of renal hilum was replaced by predominantly solid cystic friable mass measuring 10×6×4 cm with extensive areas of haemorrhage, necrosis and cystic degeneration. Adrenal was not identified grossly [Table/Fig-2].
CECT abdomen: a well defined heterogeneous mass replacing the right kidney displacing the surrounding structures.

Gross specimen: kidney measuring 14×8×6 cm with rupture at upper pole and lateral wall, replaced by predominantly solid cystic friable mass with areas of haemorrhage and necrosis.

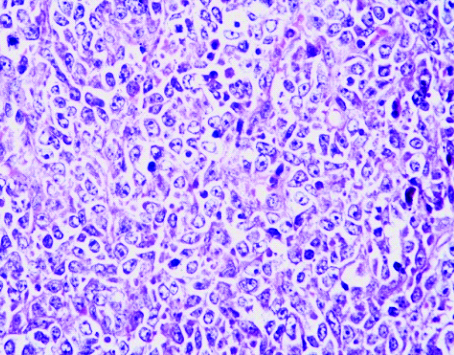
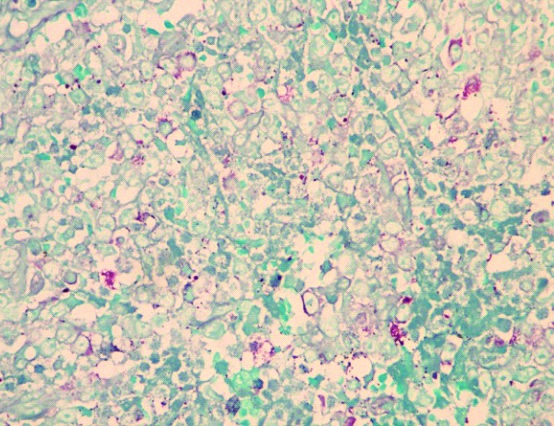
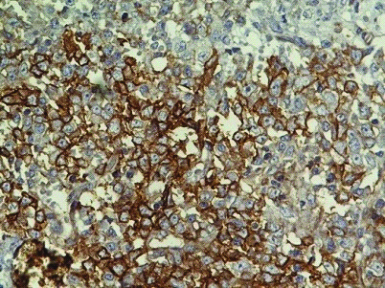
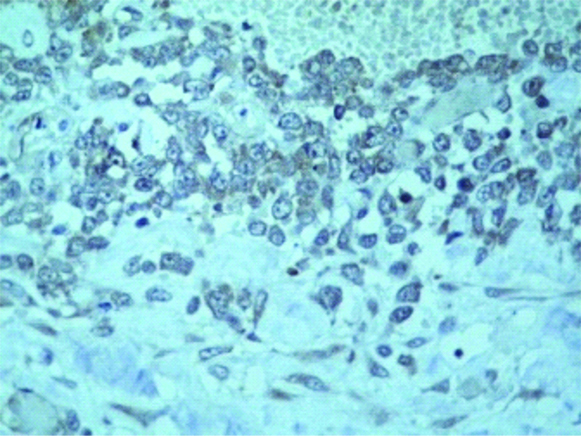
Multiple sections examined from the mass showed well circumscribed unencapsulated tumour separated into multiple nodules by thin fibrous septa with areas of extensive haemorrhage and necrosis. The tumour was composed of large round cells with moderate amount of clear to eosinophilic cytoplasm, round to irregular nucleus, moderate to marked nuclear pleomorphism, coarse granular chromatin and prominent nucleolus [Table/Fig-3a,b]. Multiple sections from the kidney did not show any blastemal component, mesenchymal component or abortive tubule. Hence, Wilms tumour was ruled out. No rosetting noted. Mitotic figures were 3-4/10 high power fields. Microscopically, tumour was seen reaching the adrenal gland and compressing the adrenal cortex. Renal sinus, hilar vessels, pelvicalyceal system was free of tumour. Proximal cut end of ureter was free of tumour. Differential diagnosis of rhabdoid tumour, Large cell lymphoma, malignant melanoma, rhabdomyosarcoma, atypical ES was considered. PAS staining for intracytoplasmic glycogen revealed an occasional cell with minimal glycogen [Table/Fig-4]. An Immunohistochemistry (IHC) panel was put for Epithelial Membrane Antigen (EMA), vimentin, CK, Leukocyte Common Antigen (LCA), HMB45, desmin, myogenin, Smooth Muscle Antigen (SMA), CD99, NSE, chromogranin, S100, WT1 and CD10. The cells showed a strong and diffuse membranous positivity for CD99, with focal positivity for NSE, vimentin and CK [Table/Fig-5a,b,c,d]. All other markers were negative. Based on the morphology and IHC results and no lump elsewhere, a final diagnosis of primary large cell (atypical) ES/PNET of kidney was given. Molecular testing for the EWS-FLI-1 fusion gene or IHC for Friend Leukemia Virus Integration-1 (FLI-1) expression could not be performed due to non-availability.
Tumour with normal kidney (H&E; 10X).

The tumour composed of round cells with moderate amount of clear to eosinophilic cytoplasm, round to irregular nucleus, moderate to marked nuclear pleomorphism, coarse granular chromatin and prominent nucleoli. (40X)
PAS positive cytoplasmic granules (H&E, 40X).
Tumour cells, CD99 positive.

Focal Vimentin positive tumour cells.

The patient was given postoperative three cycles of chemotherapy with no evidence of metastasis or recurrence till date.
Discussion
The ES/PNET is a group of rare, primitive, biologically aggressive tumours derived from the neuroectoderm. It typically arises in the soft tissue and bones of children and young adults [1]. Primary ES/PNET of the kidney is an exceptionally rare malignant lesion [2]. Median age of presentation is 27 years. It is extremely uncommon in less than 15 years. Only a few sporadic cases have been reported so far [3]. The majority of patients present with back pain (most common) and haematuria [4]. Large-cell (atypical) Ewing’s tumour differed from the classic Ewing tumour in having larger, more pleomorphic cells, often with conspicuous nucleoli. These cases were reported in bone [5]. Large cell (atypical) variants, with abundant eosinophilic cytoplasm, large irregular nuclei, vesicular chromatin, and prominent eosinophilic nucleoli have been described by Renshaw AA et al., [6]. There are a series of histological changes in this family of tumour, but the precise criteria for designating a tumour as ES or PNET were less critical. It is now recognised that both ES and PNET represent a morphologic spectrum of the same underlying process [1]. After extensive search of the literature, we could not find any case of primary large cell (atypical) ES/PNET of kidney.
Atypical ES Family of Tumour (ESFT) are the most challenging of the ESFT subtypes, and the differential diagnosis with other tumours of bone and soft tissue is difficult since these subtypes can resemble other neoplasms [7]. The present case highlights the occurrence of a renal mass in a 10-year-old girl which clinically was considered as Wilms tumour or renal cell carcinoma. However, on histology, no blastemal, mesenchymal or stromal component was seen in the multiple sections taken from the tumour. The cells were instead large round with moderate amount of clear to eosinophilic cytoplasm, round to irregular nucleus, moderate to marked nuclear pleomorphism, coarse granular chromatin and prominent nucleolus with extensive areas of haemorrhage and necrosis. No rosetting was noted. Differential diagnosis of large cell renal tumours in this age group to be considered are rhabdoid tumour, Large cell lymphoma, malignant melanoma, rhabdomyosarcoma, atypical ES.
Histologically, rhabdoid tumour show sheets of large cells with nucleus showing central nucleolus and eosinophilic cytoplasm. Mitosis, apoptosis, areas of haemorrhage and necrosis are present. Tumour cells show positive staining with vimentin and EMA [8]. Large cell lymphoma reveals monomorphic cells of lymphoid lineage in sheets with large vesicular nuclei and prominent nucleoli. These cells are positive for LCA [9]. Malignant melanoma also show large cells with vesicular nuclei, prominent nucleoli and eosinophilic cytoplasm and are positive for HMB45 [10]. Rhabdomyosarcoma composed of characteristic, round-to-oval, eosinophilic rhabdomyoblasts in diffuse sheets and show positivity for desmin, muscle-specific actin, myoglobin, MyoD1 and myogenin [11]. Atypical cases of ES/PNET have been reported to have the immunophenotype of ES/PNET, including reactivity for CD99 [7]. In present case, PAS staining for intracytoplasmic glycogen revealed minimal glycogen in an occasional cell. An IHC panel showed a strong and diffuse membranous positivity for CD99, with focal positivity for vimentin, NSE and CK. All other markers were negative. Vimentin and NSE have been reported positive in more than 80% of cases of ES/PNET [12,13]. Based on the morphology and IHC results, a final diagnosis of primary renal large cell (atypical) ES/PNET was considered. The FLI-1 is a DNA-binding transcription factor which has been found to be over expressed in a majority of ES/PNET [14]. Because of the rarity of this tumour, there is no standardised treatment strategy. The primary modality is surgical excision. The five year survival rate for patients treated with chemotherapy has been reported at 45% to 55% [1].
Conclusion
Although atypical ES/PNET is rare, ancillary studies are recommended to establish the correct diagnosis. The present case report highlights rare occurrence of primary renal large cell (atypical) ES/PNET in a child and importance of including it under the differential diagnosis.
[1]. Celli R, Cai G, Ewing sarcoma/primitive neuroectodermal tumour of the kidney. A rare and lethal entityArch Pathol Lab Med 2016 140:281-85.10.5858/arpa.2014-0367-RS26927724 [Google Scholar] [CrossRef] [PubMed]
[2]. Maeda M, Tsuda A, Yamanishi S, Uchikoba Y, Fukunaga Y, Okita H, Ewing sarcoma/primitive neuroectodermal tumour of the kidney in a childPediatr Blood Cancer 2008 50:180-83.10.1002/pbc.2083116544300 [Google Scholar] [CrossRef] [PubMed]
[3]. Aghili M, Rafiei E, Mojahed M, Zare M, Renal primitive neuroectodermal tumour: Does age at diagnosis impact outcomes?Rare Tumours 2012 4(1):e1510.4081/rt.2012.e1522532913 [Google Scholar] [CrossRef] [PubMed]
[4]. Risi E, Iacovelli R, Altavilla A, Alesini D, Palazzo A, Mosillo C, Clinical and pathological features of primary neuroectodermal tumour/Ewing sarcoma of the kidneyUrology 2013 82(2):382-86.10.1016/j.urology.2013.04.015 [Google Scholar] [CrossRef]
[5]. Nascimento AG, Unii KK, Pritchard DJ, Cooper KL, Dahlin DC, A clinicopathologic study of 20 cases of large-cell (atypical) Ewing’s sarcoma of boneAm J Surg Pathol 1980 4(1):29-36.10.1097/00000478-198004010-000037361993 [Google Scholar] [CrossRef] [PubMed]
[6]. Renshaw AA, Perez-Atayde AR, Fletcher JA, Granter SR, Cytology of typical and atypical Ewing’s sarcoma/PNETAm J Clin Pathol 1996 106:620-24.10.1093/ajcp/106.5.620 [Google Scholar] [CrossRef]
[7]. Machado I, Noguera R, Mateos EA, Calabuig-Farinas S, López FI, Martimez A, The many faces of atypical Ewing’s sarcoma. A true entity mimicking sarcomas, carcinomas and lymphomasVirchows Arch 2011 458(3):281-89.10.1007/s00428-010-1023-421181413 [Google Scholar] [CrossRef] [PubMed]
[8]. Shukla D, Pradhan A, Bhardwaj M, Malhotra V, Malignant rhabdoid tumour of kidney and brain in an infantIndian Pediatrics 2015 52:65-66.10.1007/s13312-015-0570-9 [Google Scholar] [CrossRef]
[9]. Das RN, Dasgupta S, Mondal S, Saha D, Chatterjee U, Diffuse large B-cell lymphoma of the kidney: a rare neoplasmIndian Journal of Pathology and Microbiology 2013 56(4):449-52.10.4103/0377-4929.12536824441246 [Google Scholar] [CrossRef] [PubMed]
[10]. Ornellas LC, Lanzoni VP, Toledo CF, Malignant melanoma with liver and spleen metastases: case reportSao Paulo Med J/Rev Paul Med 2000 118(2):53-56.10.1590/S1516-3180200000020000610772698 [Google Scholar] [CrossRef] [PubMed]
[11]. Mondal K, Mandal R, Congenital anaplastic rhabdomyosarcoma presenting as abdominal wall massIran J Pathol 2016 11(1):80-84. [Google Scholar]
[12]. Ellinger J, Bastian PJ, Hauser S, Biermann K, Muller SC, Primitive neuroectodermal tumour: rare, highly aggressive differential diagnosis in urologic malignanciesUrology 2006 68(2):257-62.10.1016/j.urology.2006.02.03716904430 [Google Scholar] [CrossRef] [PubMed]
[13]. Marley EF, Liapis H, Humphrey PA, Primitive neuroectodermal tumour of the kidney-another enigma: a pathologic, immunohistochemical, and molecular diagnostic studyAm J Surg Pathol 1997 21(3):354-59.10.1097/00000478-199703000-00013 [Google Scholar] [CrossRef]
[14]. May WA, Lessnick SL, Braun BS, The Ewing’s sarcoma EWS/FLI-1 fusion gene encodes a more potent transcriptional activator and is a more powerful transforming gene than FLI-1Mol Cell Biol 1993 13(12):7393-98.10.1128/MCB.13.12.7393PMC364810 [Google Scholar] [CrossRef] [PubMed]