Periodontal diseases are chronic inflammatory diseases, multifactorial in nature, characterised by destructive inflammatory processes affecting the supporting structures of the teeth, leading to the destruction of connective tissue and alveolar bone [1,2].
The pathogenesis of periodontal destruction involves a complex interaction between bacterial pathogens and the host tissues [3]. Though, microbial elements of dental plaque are the initiating factors, much of the periodontal tissue destruction observed is host-mediated, through release of inflammatory cytokines by periodontal tissue cells and immune cells [2,4]. Amongst the various inflammatory cytokines Interleukin (IL) -1, IL-6 and TNF-α play a central role in destruction of periodontal tissues [3].
The TNF-α is a pro-inflammatory and immunoregulatory cytokine released by T-lymphocytes, activated monocytes and macrophages and promotes critical inflammatory responses during periodontal disease [5,6]. It leads to tissue destruction by stimulation of bone resorption and induction of other cytokines, inflammatory mediators and tissue degrading enzymes [7,8]. Thus, while bacteria are the initiating agents in periodontitis, the host response to the pathogenic infection is critical to disease progression [9].
The homeostasis of the periodontium involves multifarious relationships, in which the endocrine system has a significant role. Endogenous sex steroid hormones have shown to orchestrate the periodontal tissue responses to microbial plaque influencing the periodontium at different stages of life such as puberty, menstruation, pregnancy and post-menopause [10].
Menopause is associated with a rapid decline in levels of circulating oestrogen which provokes disregulation of the remodelling sequence of periodontal tissue affecting the balance between bone formation and resorption by modulating the production of inflammatory cytokines [4,11]. Clinical observations have established an increased prevalence of periodontal diseases in post-menopausal women with lower oestrogen levels, even when oral hygiene was maintained [4,12]. The key mechanism proposed is by up-regulation of the production of TNF-α [13,14]. Thus, increase in the levels of TNF-α is regarded as a key causative factor in the bone loss seen in oestrogen deficient post-menopausal women.
Early diagnosis of periodontal disease is fundamental in preventing complications that could negatively affect the quality of a patient’s life [15]. Periodontal diseases have high prevalence worldwide and incur enormous economic and social consequences. Their treatment is not only time consuming but also lacks certainty in predicting outcome and stability. Thus, screening and diagnostic modalities for risk determination, early identification of initiation and progression of periodontitis, as well as objective measures for monitoring response to therapy, are being sought [5,11].
Whole saliva represents a promising diagnostic fluid for the screening of periodontal disease. The revelation that saliva contains molecular profiles that mirrors diseases in the body has led to a new non-invasive diagnostic methodology of salivary diagnostics [16]. Several mediators of chronic inflammation have been detected in saliva of patients with clinical indicators of periodontitis [5].
To the best of present information no authoritative study has been conducted comparing the levels of salivary TNF-α in pre- and post-menopausal women with chronic periodontitis.
Thus, the present study was conducted to assess the levels of salivary TNF-α in pre- and post-menopausal women with chronic periodontitis and evaluate its potential as a biochemical marker of periodontal disease in post-menopausal women.
Materials and Methods
The study was a double-blind, case-control clinical study. It was conducted in the Department of Periodontics, KLE VK. Institute of Dental Sciences, KLE University, Belgaum, Karnataka, India. The Institutional Ethical Committee of KLE VK. Institute of Dental Sciences, Belgaum, India approved the study and it was conducted in full accordance with ethical principles for research involving human subjects outlined in World Medical Association Declaration of Helsinki (version 2008). Prior to starting the study, a written informed consent was obtained from all the participants.
A total of 80 female subjects, within the age range of 35-55 years, were divided into 4 groups of 20 subjects each, pre- and post-menopausal women with chronic periodontitis (Test groups) and pre- and post-menopausal periodontally healthy controls (Control groups) [Table/Fig-1].
Age distribution of the study groups (Mean±SD).
| Group I | Group II | Group III | Group IV |
|---|
| n | 20 | 20 | 20 | 20 |
| Age (years) | 38.75±3.14 | 50.15±3.67 | 38.50±3.33 | 50.55±3.17 |
| Age difference |
| I and III | II and IV |
| (p-value) | 0.809* | 0.715* |
n=Sample size; *Non-significant, p<0.05: significant
Inclusion criteria for the test groups was systemically healthy females with existing moderate to severe chronic generalised periodontitis with radiographic evidence of alveolar bone loss. According to the American Academy of Periodontology Task Force Report 2015 subjects with moderate to severe periodontitis exhibited probing depths ≥5 mm and clinical attachment loss ≥3 mm. The control groups included subjects with clinically healthy gingiva that did not bleed on probing with probing depth ≤3 mm and no clinical attachment loss.
Pre-menopausal women within the age range of 35-45 years and post-menopausal women within the age range of 45-55 years were included. Post-menopausal women were within five years of menopause and had attained a natural menopause.
Patients with a history of smoking and/or alcoholism, suffering from systemic diseases/illness, with any oral mucosal inflammatory condition, had taken any anti-inflammatory drugs, antibiotics, nutritional supplements or any other drugs or had under gone any periodontal treatment over the past six months were excluded from the study. Post-menopausal women who had received Hormone Replacement Therapy (HRT) and pre-menopausal women in menstruation period or who had received hormonal treatment, were using oral contraceptives or were pregnant or lactating were excluded.
Collection of Whole Unstimulated Saliva
Prior to clinical measurements, 2 mL of unstimulated whole saliva was collected. Subjects were seated in upright position and asked to spit into sterile containers after rinsing their mouth with tap water. The samples were immediately centrifuged and the supernatant was aliquoted. Aliquots were freezed at -80°C until assayed.
Clinical Examination
Periodontal examination was performed by a single trained periodontist. Assessment of oral hygiene and gingival status were done using simplified Oral Hygiene Index (OHIS) (Greene and Vermillion 1964), Gingival Index (GI) (Löe and Silness, 1963) and Gingival Bleeding Index (GBI) (Ainamo and Bay 1975).
Full mouth periodontal Probing Depth (PD) and Clinical Attachment Loss (CAL) were determined on six sites per tooth (mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual and disto-lingual) using a graduated UNC 15 probe.
Assay Procedure
The TNF-α level in saliva were determined by using a quantitative sandwich ELISA kit. (Quantikine Human TNF-α Immunoassay, R&D Systems, Minneapolis, MN, USA.)
The assay employed the quantitative sandwich enzyme immunoassay technique. A monoclonal antibody specific for TNF-α was pre-coated onto a microplate. Standards and samples were pipetted into the wells and any TNF-α present was bound by the immobilised antibody. After washing away any unbound substances, an enzyme-linked polyclonal antibody specific for TNF-α was added to the wells. After removing the unbound antibody-enzyme reagent with a wash, a substrate solution was added to the wells. Colour was developed in proportion to the amount of the bound TNF-α and the intensity of the colour was then measured.
Statistical Analysis
Student’s unpaired t-test was used to assess the age distribution between the pre- and post- menopausal women in the chronic periodontitis and the control groups. The mean values of the clinical parameters and salivary TNF-α levels were assessed by a one-way Analysis of variance (ANOVA). The pair-wise comparison was done using post-hoc Tukey’s test. Mean salivary TNF-α levels were compared between pre- and post-menopausal healthy controls and chronic periodontitis groups using Student’s unpaired t-test. Karl Pearson’s co-efficient of correlation was used to assess associations between levels of salivary TNF-α level and the clinical parameters. A p-value <0.05 was considered as statistically significant. The Statistical Package for Social Sciences Software (SPSS) version 17.0 (Chicago: SPSS Inc.) and MedCalc Statistical Software version 10.2 (MedCalc Software bvba, Ostend, Belgium) were used to perform the data analyses.
Results
The study consisted of 80 participants with 20 subjects each in the 4 study groups. No statistically significant difference was found with respect to mean age between both the pre-menopausal groups as well as between both the post-menopausal groups [Table/Fig-1].
Comparison of the various clinical parameters (OHI-S, GI, GBI, PD and CAL) between the study groups revealed that while all the clinical parameters were significantly higher for subjects with chronic periodontitis than healthy controls (p<0.05), there was no significant difference between pre- and post-menopausal women with chronic periodontitis as well as between pre- and post-menopausal healthy controls (p>0.05) [Table/Fig-2,3]. This indicated both the periodontitis groups as well as the healthy control groups were comparable with respect to their periodontal status.
Clinical parameters and salivary TNF-α levels of the study groups.
| Group I | Group II | Group III | Group IV | One-way ANOVA test |
|---|
| n | 20 | 20 | 20 | 20 | p-value |
| OHI-S | 0.20±0.15 | 0.26±0.16 | 3.77±0.84 | 3.74±0.79 | <0.001 |
| GI | 0.04±0.04 | 0.06±0.04 | 1.76±0.13 | 1.81±0.13 | <0.001 |
| GBI (% of sites involved) | 0 | 0 | 75.24±12.97 | 80.02±12.67 | <0.001 |
| PD (mm) | 1.74±0.70 | 1.80±0.68 | 5.69±0.46 | 5.84±0.45 | <0.001 |
| CAL (mm) | 0 | 0 | 5.98±0.55 | 6.11±0.52 | <0.001 |
| TNF-α (pg/mL) | 2.05±1.11 | 2.40±1.08 | 4.25±1.20 | 5.13±1.35 | <0.001 |
n=Sample size, p<0.001: Very significant
OHI-S: Oral hygiene index; GI: Gingival index; GBI: Gingival bleeding index; PD: Probing depth; CAL: Clinical attachment loss; TNF-α: Tumor necrosis factor-alpha
Comparison of clinical parameters and salivary TNF-α levels between the study groups (Mean±SD).
| | I and II | I and III | I and IV | II and III | II and IV | III and IV |
|---|
| OHI-S | Post-hoc Tukey’s test (p-value) | 0.993* | <0.001 | <0.001 | <0.001 | <0.001 | 0.998* |
| GI | 0.978* | <0.001 | <0.001 | <0.001 | <0.001 | 0.326* |
| GBI | 1.00* | <0.001 | <0.001 | <0.001 | <0.001 | 0.347* |
| PD | 0.988* | <0.001 | <0.001 | <0.001 | <0.001 | 0.840* |
| CAL | 1.00* | <0.001 | <0.001 | <0.001 | <0.001 | 0.719* |
| TNF-α | 0.788* | <0.001 | <0.001 | <0.001 | <0.001 | 0.041 |
*Non-Significant; p <0.001: Very Significant
OHI-S: Oral hygiene index; GI: Gingival index; GBI: Gingival bleeding index; PD: Probing depth; CAL: Clinical attachment loss; TNF-α: Tumor necrosis factor-alpha
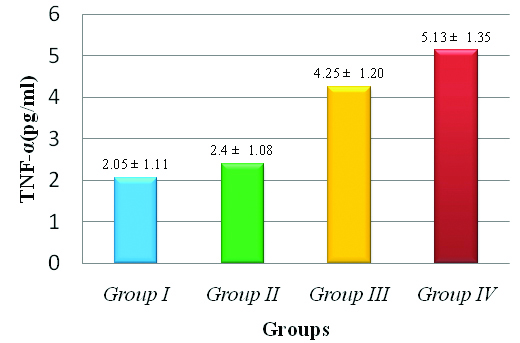
The mean level of salivary TNF-α in pre- and post-menopausal women with chronic periodontitis were 4.25 pg/mL and 5.13 pg/mL, respectively, whereas that in pre- and post-menopausal healthy controls were 2.05 pg/mL and 2.40 pg/mL, respectively.
Although, the mean value of salivary TNF-α levels was higher in post-menopausal healthy controls than pre-menopausal healthy controls, however the difference was statistically non-significant (p=0.3195). Statistically significant difference was observed in salivary TNF-α levels between pre- and post-menopausal women with chronic periodontitis (p=0.0362), with the levels being higher in post-menopausal women [Table/Fig-3,4, and 5].
Comparison of salivary TNF-α levels (Mean±SD) between pre- and post-menopausal women in health and chronic periodontitis (Mean±SD).
| Group I | Group II | Group III | Group IV |
|---|
| TNF-α (pg/mL) | 2.05±1.11 | 2.40±1.08 | 4.25±1.20 | 5.13±1.35 |
| Student’s unpaired t-test (p-value) | 0.3195* | 0.036 |
p<0.05: Significant;* Non-significant; TNF-α: Tumor necrosis factor-alpha
Comparison of salivary TNF-α levels between the study groups (Mean±SD).
There was a strong positive correlation between salivary TNF-α levels and all the assessed clinical parameters (OHI-S, GI, GBI, PD and CAL) (p<0.001) [Table/Fig-6].
Karl Pearson’s Correlation (r) analysis between salivary TNF-α levels and clinical parameters in all the study groups (Mean±SD).
| Salivary TNF-α levels |
|---|
| r | p-value |
|---|
| OHI-S | 0.8075 | <0.001 |
| GI | 0.7647 | <0.001 |
| GBI | 0.8005 | <0.001 |
| PD | 0.8148 | <0.001 |
| CAL | 0.7522 | <0.001 |
p<0.001: Very Significant
OHI-S: Oral hygiene index; GI: Gingival index; GBI: Gingival bleeding index; PD: Probing depth; CAL: Clinical attachment loss
Discussion
The growing recognition of the clinical, biological and microbiological relationship of periodontitis with systemic health and disease has revealed a novel approach to present understanding of pathogenesis of periodontal disease and its potential clinical applications [15,16].
The role of inflammation and immune dysregulation are well established in the pathogenesis of periodontitis. Initial response to bacterial infection is a local inflammatory reaction that activates the innate immune system, amplification of which results in the release of an array of cytokines and other inflammatory mediators, thus orchestrating the pathogenesis of periodontal diseases and concomitant periodontal destruction [17-19].
TNF-α is one of the primary mediators of inflammation that stimulates the expression of other mediators, such as chemokines and prostaglandins, amplify the inflammatory response and leads to production of lytic enzymes such as MMPs that degrade connective tissue. It has synergistic capacity with IL-1 to enhance bone resorption. It may also limit repair of periodontal tissues by inducing apoptosis of matrix-producing cells [17,20]. It has been established that the activity of TNF-α coincides with the critical events that occur during periodontal disease, namely loss of attachment and bone resorption [20].
Increased level of TNF-α is also regarded as an important causal factor of the bone loss evident in post-menopausal women with oestrogen deficiency. The present study assessed the levels of salivary TNF-α in pre- and post-menopausal women with chronic periodontitis.
The results of the present study revealed salivary TNF-α levels were significantly elevated in subjects with chronic periodontitis when compared to healthy subjects (p<0.001). This is in concurrence with the results of the studies done by Rai B et al., and Biloklytska HF et al., [21,22]. Levels of salivary TNF-α also showed a significant positive correlation with all the assessed clinical parameters (p<0.001). The study conducted by Frodge BD et al., corroborates this finding [5].
In various clinical studies, increased levels of TNF-α in biological fluids and gingival tissue samples from periodontitis sites have been associated with gingival inflammation, and severity of periodontitis and the levels have shown a decline post periodontal therapy [5,21,23-30]. Present detection of elevated levels of TNF-α in subjects with chronic periodontitis and its correlation with the clinical features of periodontal disease, was consistent with the cytokine’s role in inflammation, periodontal tissue destruction and bone resorption and suggests that TNF-α is an important part of the panel of salivary biomarkers for screening patients for periodontal disease.
In the present study, we also compared the levels of salivary TNF-α in pre- and post-menopausal women. The results indicated that salivary TNF-α levels were significantly higher in post menopausal women with chronic periodontitis than the pre-menopausal group with comparable periodontal destruction (p=0.0362). The mean value of salivary TNF-α levels was also higher in post-menopausal healthy controls than the pre-menopausal healthy controls, however this difference was statistically non-significant (p=0.3195). These findings imply that such a difference in the levels of salivary TNF-α may be related to hormonal status rather than periodontal status alone.
The concentration of circulating oestrogen declines after menopause. Oestrogen has shown to inhibit pro-inflammatory cytokines release by human marrow cells, inhibit neutrophil chemotaxis, suppress leukocyte production from the bone marrow, reduce T-cell mediated inflammation, stimulate the proliferation of gingival fibroblasts and synthesis and maturation of gingival connective tissue. Consequently in post-menopausal women the decline in the circulating oestrogen levels compromises the anti-inflammatory effect of this hormone on the periodontium [10,31].
Thus, it is the rapid decline in circulating oestrogen associated with menopause that leads to increase in the expression and secretion of the pro-inflammatory cytokines like IL-1, IL-6 and TNF-α. Genco and Grossi (1998) proposed a model according to which oestrogen deficiency leads to increased production of pro-inflammatory cytokines by osteoblasts and immune cells, when challenged by products related to periodontal bacteria. This starts the inflammation cascade and leads to activation of tissue proteinases and degradative enzymes, leading to connective tissue destruction, resorption of alveolar bone and ultimately tooth loss which can explain the enhanced risk of periodontal disease in post-menopausal women [11,32].
Several studies have linked menopause with periodontal conditions [8,33-36].
Most of the effects of oestrogen on cytokine activity occur by activating the oestrogen receptors found on periosteal fibroblasts, periodontal ligament fibroblasts, scattered fibroblasts of the lamina propria, and osteoblasts [10,31]. It has been established that up-regulation of TNF-α production by activated T-cell is the key mechanism by which oestrogen deficiency in post menopausal women induces bone loss [11,14].
Oestrogen deficiency up-regulates T-cell TNF-α production by increasing T-cell activation which in turn results in enhancing T-cell proliferation and life span. The decreased expression of Transforming Growth Factor-Beta (TGF-β) associated with decreased oestrogen levels leads to enhanced expression of Interferon-Gamma (IFN-γ) and subsequent augmented expression of major histocompatibility complex II which eventually leads to enhanced antigen presentation and thus, T-cell activation. Oestrogen deficiency also up-regulates the production of IL-17 which may directly stimulate T-cell proliferation or increase antigen presentation by increasing the production of IFN-γ or inversely regulating the production of TGF-α [14,37]. The rise in the production of Follicular Stimulating Hormone (FSH) due to oestrogen deficiency also stimulates the secretion of TNF-α [14]. The findings of various studies have demonstrated the key causal role of TNF-α in mediating the effects of oestrogen deficiency on bone [11,13,14,38,39].
Progesterone is another sex hormone that declines with menopause subsequently increasing human macrophage and monocyte IL-1 and TNF-α production [11]. The post-menopausal decline in the concentration of circulating androgens which are the major source of oestrogen after menopause, may also up-regulate pro-inflammatory cytokines, in a way similar to oestrogen deficiency [11,31].
The non-significant increase in the salivary TNF-α levels in post-menopausal healthy controls as compared to pre-menopausal healthy controls can be explained by the fact that there is only a subtle spontaneous increase in the secretion of the pro-inflammatory cytokines with oestrogen deficiency in post menopausal women when compared with the increase observed as a host reaction to infection or tissue injury [11]. Oestrogen deficient women have shown increased production of bone-resorbing cytokines by immune cells only when challenged by products related to bacterial plaque biofilm, lipopolysaccharides and toxin, emphasising on the necessity of bacteria to initiate the host’s inflammatory response [40]. Menopause may affect the severity of the present disease, however post menopausal women in good gingival health cannot be considered to be at increased risk of periodontal disease [41].
Analysis of salivary biomarkers offers various advantages. As whole saliva represents a pooled sample from all periodontal sites, it gives an overall assessment of disease status. Levels of salivary analytes may reflect current disease activity as well as severity and may offer a way of assessing subject-level risk or status. Also, collection of whole saliva is easy, non-invasive, rapid and requires no special equipment or expertise [42,43]. All these characteristics make saliva a promising diagnostic modality for monitoring of several biomarkers in infants, children, adults and uncooperative patients. With the development of increased sensitivity of detection techniques, the most often quoted criticism of low levels of salivary analytes has been overcome.
Limitation
The present study was a cross-sectional study where the effect of periodontal treatment on salivary levels of TNF-α was not assessed. Thus, further long term, rigorous, multicentric longitudinal clinical studies should be conducted with larger sample size to verify the relationship between specific biomarkers with different stages of progression of periodontal disease in post menopausal women. The effect of periodontal therapy on the salivary levels of TNF-α should be assessed to elucidate the therapeutic effects of therapy on the levels of salivary biomarkers in post-menopausal women.
Conclusion
Increased secretion of TNF-α in post-menopausal women could be implicated as a contributing factor to the progression of periodontal disease, suggesting that levels of salivary TNF-α may serve as a candidate biomarker of periodontal disease in post-menopausal women.
With the advent of the point of care testing due to the amalgamation of advances in biotechnology and the salivary diagnostics, risk determination especially in systemically compromised individuals, early diagnosis, intervention and prevention of complications of advanced diseases may become a reality soon in the medical and dental care settings.
n=Sample size; *Non-significant, p<0.05: significant
n=Sample size, p<0.001: Very significant
OHI-S: Oral hygiene index; GI: Gingival index; GBI: Gingival bleeding index; PD: Probing depth; CAL: Clinical attachment loss; TNF-α: Tumor necrosis factor-alpha
*Non-Significant; p <0.001: Very Significant
OHI-S: Oral hygiene index; GI: Gingival index; GBI: Gingival bleeding index; PD: Probing depth; CAL: Clinical attachment loss; TNF-α: Tumor necrosis factor-alpha
p<0.05: Significant;* Non-significant; TNF-α: Tumor necrosis factor-alpha
p<0.001: Very Significant
OHI-S: Oral hygiene index; GI: Gingival index; GBI: Gingival bleeding index; PD: Probing depth; CAL: Clinical attachment loss