Bacteria and fungi are omnipresent in the habitat such as soil, water, air, etc. Microbes associated with infections are present in patient’s endogenous system, healthcare equipment’s and environment. The nosocomial infection occurs due to the microbes present in the environment which is a major source of infection to mother and newborn baby in maternity homes [1]. Pseudomonas aeruginosa and Bacillus cereus from bacteria and filamentous fungi, Mucor and Aspergillus spp. are most commonly found in the hospital and operation theatres [2-4]. For this reason, assessment of the microbial contamination is of greater concern. As the chances of infection are more, due to patient condition and exposure to air [5]. Thus, assessment and maintenance of environmental hygiene is needed in order to reduce the chance of postoperative infections [6].
These maternity homes have the complex infrastructure, whereas other factors like overcrowding, inadequate design and aeration facilitate the growth of microbes [7]. On the other hand, climatic conditions like high humidity, moisture on the wall and ceiling aid in fungal growth. Physical parameters such as temperature and humidity play the crucial role in survival and development of microbes [8]. The dry, wet and moderate humid climatic conditions prevail in the Moga region, Punjab, India. Thus, assessment of microbial contamination in the air with respect to change climatic conditions is needed. The air sampling enlightens about the microbes that endure in the environment and cause severe infection in patients [9]. The present study was rationalised to check the presence of the air-microbes in the maternity homes that can be potent to induce nosocomial infection. Passive method of sampling is stated as an effective method for the risk assessment as it enables us to measure the harmful microbes, which colonise on medical, surgical instruments [10]. The aim of the present study was to assess the microbial contamination (aerobic bacteria, facultative anaerobic bacteria, and fungi) load, by employing passive sampling procedure and characterisation of the microbes that sustain in the indoor air of the private maternity homes.
Materials and Methods
Experimental Design
The present prospective experiment was designed to assess the microbial contamination persisting in the private maternity home. The study was conducted at Baba Isher Singh College of Engineering and Technology and the sampling was done in five different private maternity homes in the province of the Moga in Punjab, India, for one month starting from December 2016 to January 2017. The Indoor air samples were collected in duplicates, twice a day (Morning (8:00-9:00 am) and Afternoon (1:00-2:00 pm) for one month by passive sampling method [11]. The sample were investigated for the different microorganisms and their load. The written permission from all the maternity homes for sampling was taken.
Area of study: The sampling sites were in the provinces of Moga of Longitude (75.2479° E) and Latitude (30.6874° N) with an elevation of 217 m from the sea level. On assessing the maternity homes for the sampling, the general ward (Average area=60±5.86 m2) and private ward (Average area=15±2.58 m2) were selected. The average number of employee in the private maternity home was 10-20 whereas the bed capacity was around 15-20 in each maternity home. All the general wards (each maternity home) were without air-conditioner, on the other hand, five private rooms were having air-conditioner and six to nine rooms were without air-conditioner but supplemented with ceiling fans for proper ventilation. Monthly fumigation was done in all the maternity homes with formaldehyde (0.1 ppm) in the last week (i.e., 22-29 of each month).
Pre-analysis and sample collection: Pre-analysis was done before the sampling every time for collecting the information of the environmental condition (temperature and humidity) with the help of hygrometer (kept 15 minute prior to sampling for adapting to the room condition) in the both General Ward (GW) and Private Ward (PW), human activity (talking, movement of sweepers, patients and their near ones) and the total number of people in the room. After, computing the data, the air sample were collected (in duplicate) by employing the passive air sampling protocol in the indoor of GW and PW for assessing the microbial load. Passive air sampling was done in which the 9 cm (diameter) petri plates were placed in 1/1/1 system (for 1 hour, 1 m from the floor and 1 m away from the wall as well as other hindrances) [11]. In the present experiment, eight different media (Nutrient agar, Potato dextrose agar, Cetrimide agar, Hicrome™ bacillus agar, MacConkey agar, Mannitol salt agar and Columbia 5% sheep blood agar, Anaerobic blood agar) plates were placed following the 1/1/1 system of sampling. The nutrient agar (for bacteria) and potato dextrose agar (for fungus) plates were used for assessing the microbial load present in the air and was expressed in CFU/m3. For standard limits of the total air microbial count as Index of Microbial Air Contamination (IMA) was considered as given by Fisher for medical wards [11].
After the collection of samples, the bacterial culture plates were placed in the incubator at 37°C for 24 hour and the fungal culture plates were placed in the incubator at 25°C for five days for the growth. After, 24 hour the nutrient agar plates were observed for growth and each plate was counted for the number bacteria in the plate for calculating the CFU value of the bacteria and same was repeated for the potato dextrose agar for the fungal growth after five days. The CFU/m3 was determined, using the following equation [12]:
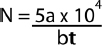
Where ‘N’ is microbial CFU/m3 of indoor air; ‘a’ is number of colonies per Petri dish; ‘b’ is dish Surface area (cm2), and ‘t’ is exposure time.
Characterisation of bacteria and fungi: The bacteria were characterised on the basis of their growth on the different media such as Cetrimide agar, Hicrome™ bacillus agar, MacConkey agar, Mannitol salt agar and Columbia 5% sheep blood agar, Anaerobic blood agar (HiMedia, Mumbai). Some species of bacteria were identified on the basis of the phenotypic traits and Gram staining [13] of the bacteria. On the other hand, the fungi were identified to their genera level by staining with Lacto-phenol cotton blue stain and by observing the morphology under the microscope [13].
Statistical Analysis
For statistical analysis SPSS version 16.0 was used to determine rank correlation coefficient by Spearman’s (where the level of significance, α=0.01) among the different concentration of bacteria and fungi evaluated at different sampling site and linearity among the different concentration of bacteria and fungi (p<0.001) evaluated at different sampling site was also assessed.
Results
The microbial loads of indoor air of five private maternity homes in the province of Moga, Punjab were investigated over a period of a month by employing passive sampling. The climatic conditions were shivery cold and humid in the morning time and moderate cold and less humid in the afternoon. On inspecting the sampling location, there was no sign of microbial growth on the ceiling and side walls of the room. The gathering of family members in GW was very low as compared to that of the in PW in the morning and conditions were vice versa in the afternoon. The physical parameters (temperature and humidity) were noted during the sampling time [Table/Fig-1]. The temperature value was ranging 17.7-23.4°C, whereas relative humidity values ranging 54-69%. [Table/Fig-1] represents the weekly distribution of the bacteria and fungi at different maternity homes in their GW and PW. Throughout the period of one month, the bacterial load was found to be high in the MH4 (ranging 1358-1552 CFU/m3 in GW and 2177-2313 CFU/m3 in PW) as compared to all the other maternity homes during the morning time. Whereas the microbial load was found to be high in the GW of the MH3 (ranging 2277-2665 CFU/m3) and PW of the MH4 (1753-1940 CFU/m3) during the afternoon time. The lowest bacterial load was observed in the MH1 during the morning time (ranging 380-510 CFU/m3 in GW and 517-625 CFU/m3 in PW) and afternoon time (ranging 732-790 CFU/m3 in GW and 259-539 CFU/m3 in PW).
Weekly distribution of bacterial and fungal CFU/m3 count at different maternity homes in their general and private ward during different time period in accordance to Fishers scheme of air total microbial count.
| Weekly | MH1 | MH2 | MH3 | MH4 | MH5 |
|---|
| General ward |
|---|
| Bacteria | | Morning(H: %, T: °C) | Afternoon(H: %, T: °C) | Morning(H: %, T: °C) | Afternoon(H: %, T: °C) | Morning(H: %, T: °C) | Afternoon(H: %, T: °C) | Morning(H: %, T: °C) | Afternoon(H: %, T: °C) | Morning(H: %, T: °C) | Afternoon(H: %, T: °C) |
|---|
| 7 days | 380*(H: 58; T: 20.1) | 783‡(H: 50; T: 21.7) | 912‡(H: 62; T: 18.9) | 1358‡(H: 66; T: 18.5) | 596†(H: 57; T: 18.8) | 2277‡(H: 56; T: 20.0) | 1552‡(H: 58; T: 18.9) | 1739‡(H: 45; T: 23.3) | 711†(H: 57; T: 20.1) | 805‡(H: 61; T: 18.8) |
| 14 days | 510†(H: 61; T: 19.8) | 732†(H: 48; T: 22.2) | 1034‡(H: 64; T: 18.5) | 1193‡(H: 57; T: 21.5) | 546†(H: 59; T: 19.2) | 2665‡(H: 54; T: 21.6) | 1487‡(H: 56; T: 19.6) | 1652‡(H: 46; T: 22.8) | 733†(H: 63; T: 17.7) | 754‡(H: 63; T: 17.2) |
| 21 days | 467†(H:57; T:20.3) | 776‡(H: 51; T: 21.5) | 1013‡(H: 59; T: 20.5) | 1293‡(H: 54; T: 22.0) | 625†(H: 61; T: 20.1) | 2356‡(H: 51; T: 21.8) | 1358‡(H: 54; T: 19.8) | 1688‡(H: 51; T: 20.3) | 754‡(H: 61; T: 18.2) | 797‡(H: 61; T: 19.3) |
| 28 days | 424*(H:60; T: 19.6) | 790‡(H: 57; T: 20.3) | 927‡(H: 63; T: 18.7) | 1013‡(H: 61; T: 19.0) | 646†(H: 59; T: 19.7) | 2277‡(H: 54; T: 21.7) | 1552‡(H: 53; T: 18.6) | 1681‡(H: 54; T: 19.8) | 769‡(H: 59; T: 18.8) | 805‡(H: 66; T: 17.8) |
| Private ward |
| 7 days | 611†(H: 69; T: 19.6) | 259*(H: 53; T: 20.7) | 690†(H: 50; T: 22.8) | 481†(H: 59; T: 20.1) | 2356‡(H: 57; T: 18.3) | 697†(H: 55; T: 19.9) | 2191‡(H: 62; T: 18.4) | 1875‡(H: 45; T: 21.6) | 783‡(H: 60; T: 19.2) | 481†(H: 64; T: 18.5) |
| 14 days | 517†(H: 64; T: 20.4) | 395*(H: 52; T: 20.9) | 790‡(H: 48; T: 23.1) | 445*(H: 61; T: 19.8) | 2205‡(H: 57; T: 18.9) | 661†(H: 58; T: 20.0) | 2198‡(H: 60; T: 20.1) | 1940‡(H: 46; T: 21.2) | 891‡(H: 62; T: 18.7) | 517†(H: 61; T: 18.9) |
| 21 days | 625†(H:59; T: 21.0) | 539†(H: 55; T: 20.3) | 733†(H: 47; T: 23.4) | 568†(H: 60; T: 20.3) | 2270‡(H: 60; T: 19.8) | 740†(H: 63; T: 18.8) | 2313‡(H: 61; T: 19.7) | 1753‡(H: 50; T: 20.8) | 848‡(H: 58; T: 19.8) | 467†(H: 56; T: 20.1) |
| 28 days | 575†(H: 65; T: 20.7) | 517†(H: 61; T: 19.5) | 826‡(H: 54; T: 21.9) | 532†(H: 54; T: 20.0) | 2270‡(H: 58; T: 18.1) | 654†(H: 65; T: 17.9) | 2177‡(H: 61; T: 19.8) | 1882‡(H: 50; T: 20.7) | 869‡(H: 60; T: 19.6) | 445*(H: 63; T: 18.7) |
| Fungi | General ward |
| 7 days | 101*(H: 58; T: 20.1) | 180*(H: 50; T: 21.7) | 79*(H: 62; T: 18.9) | 172*(H: 66; T: 18.5) | 251*(H: 57; T: 18.8) | 568†(H: 56; T: 20.0) | 345*(H: 58; T: 18.9) | 374*(H: 45; T: 23.3) | 295*(H: 57; T: 20.1) | 575†(H: 61; T: 18.8) |
| 14 days | 136*(H: 61; T: 19.8) | 244*(H: 48; T: 22.2) | 79*(H: 64; T: 18.5) | 208*(H: 57; T: 21.5) | 216*(H: 59; T: 19.2) | 582†(H: 54; T: 21.6) | 266*(H: 56; T: 19.6) | 474†(H: 46; T: 22.8) | 273*(H: 63; T: 17.7) | 754‡(H: 63; T: 17.2) |
| 21 days | 151*(H:57; T:20.3) | 158*(H: 51; T: 21.5) | 115*(H: 59; T: 20.5) | 208*(H: 54; T: 22.0) | 266*(H: 61; T: 20.1) | 647†(H: 51; T: 21.8) | 316*(H: 54; T: 19.8) | 553†(H: 51; T: 20.3) | 244*(H: 61; T: 18.2) | 826‡(H: 61; T: 19.3) |
| 28 days | 158*(H:60; T: 19.6) | 273*(H: 57; T: 20.3) | 108*(H: 63; T: 18.7) | 230*(H: 61; T: 19.0) | 230*(H: 59; T: 19.7) | 697†(H: 54; T: 21.7) | 323*(H: 53; T: 18.6) | 467†(H: 54; T: 19.8) | 316*(H: 59; T: 18.8) | 726†(H: 66; T: 17.8) |
| Private ward |
| 7 days | 431*(H: 69; T: 19.6) | 251*(H: 53; T: 20.7) | 115*(H: 50; T: 22.8) | 65*(H: 59; T: 20.1) | 172*(H: 57; T: 18.3) | 244*(H: 55; T: 19.9) | 366*(H: 62; T: 18.4) | 144*(H: 45; T: 21.6) | 330*(H: 60; T: 19.2) | 280*(H: 64; T: 18.5) |
| 14 days | 496†(H: 64; T: 20.4) | 208*(H: 52; T: 20.9) | 172*(H: 48; T: 23.1) | 57*(H: 61; T: 19.8) | 208*(H: 57; T: 18.9) | 115*(H: 58; T: 20.0) | 424*(H: 60; T: 20.1) | 122*(H: 46; T: 21.2) | 352*(H: 62; T: 18.7) | 287*(H: 61; T: 18.9) |
| 21 days | 481†(H:59; T: 21.0) | 194*(H: 55; T: 20.3) | 244*(H: 47; T: 23.4) | 100*(H: 60; T: 20.3) | 244*(H: 60; T: 19.8) | 79*(H: 63; T: 18.8) | 309*(H: 61; T: 19.7) | 136*(H: 50; T: 20.8) | 409*(H: 58; T: 19.8) | 280*(H: 56; T: 20.1) |
| 28 days | 553†(H: 65; T: 20.7) | 244*(H: 61; T: 19.5) | 223*(H: 54; T: 21.9) | 129*(H: 54; T: 20.0) | 172*(H: 58; T: 18.1) | 101*(H: 65; T: 17.9) | 467†(H: 61; T: 19.8) | 172*(H: 50; T: 20.7) | 381*(H: 60; T: 19.6) | 330*(H: 63; T: 18.7) |
MH1: Maternity home 1; MH2: Maternity home 2; MH3: Maternity home 3; MH4: Maternity home 4; MH5: Maternity home 5; *Optimal (Range-0-450; †Acceptable (range-451-750); ‡Not Acceptable (range->751); H: Humidity; T: Temperature
On the other hand, the fungal load was found to be high in the GW of the MH4 (266-345 CFU/m3) and PW of the MH1 (431-553 CFU/m3) during the morning time. Whereas, the fungal load was found to be high in the MH5 (ranging 575-826 CFU/m3 in GW and 280-330 CFU/m3 in PW) during the afternoon time. The lowest fungal load was observed in the MH2 (ranging 79-115 CFU/m3 in GW and 115-244 CFU/m3 in PW) during the morning time. Lowest fungal load was observed in the GW of the MH1 (158-273 CFU/m3) and PW of the MH2 (57-129 CFU/m3) during the afternoon time.
Characterisation of bacteria and fungi: The bacteria were characterised up to species and genera level on the basis of the Gram staining, morphology, and colour on the distinct media. On the other hand, the fungi were characterised up to species on the basis of their morphology and staining it with Lacto-phenol cotton blue stain. The isolated microbes include Bacillus spp.,Staphylococcus spp.,Pseudomonas spp.,Enterobacteriaceae spp.; haemolytic, Gram negative rods; non haemolytic, Gram positive rods; non haemolytic, Gram positive cocci; facultative anaerobic, haemolytic, Gram negative rods; and facultative-anaerobic, non-haemolytic, Gram-negative rods in the case of bacteria. Whereas, Aspergillus spp., Mucor spp., Rhizopus spp., Penicillium spp., Exophiala spp., and Absidia spp. in case of fungi. The type and their distribution among the different maternity homes are illustrated in [Table/Fig-2].
Types of microorganism isolated and their distribution among the different maternity homes.
| Type of microorganism’s isolate | MH1 | MH2 | MH3 | MH4 | MH5 |
|---|
| GW | PW | GW | PW | GW | PW | GW | PW | GW | PW |
|---|
| Bacteria |
| Bacillus cereus | - | + | + | - | + | + | + | + | - | - |
| Bacillus subtilis | + | + | + | + | + | + | + | - | + | + |
| Staphylococcus spp. | + | + | + | + | + | + | + | + | + | + |
| Pseudomonas spp. | + | + | - | + | + | + | + | + | - | - |
| Enterobacteriaceae spp. | + | + | + | + | - | - | - | - | | |
| Haemolytic Gram negative rods | + | + | + | + | + | + | + | + | + | + |
| Non-haemolytic Gram positive rods | + | + | - | + | - | + | - | - | + | + |
| Non-haemolytic Gram positive cocci | + | + | + | + | - | + | + | + | + | + |
| Facultative anaerobic haemolytic Gram negative rods | + | + | + | + | + | + | + | + | + | + |
| Facultative anaerobic Non- haemolytic Gram negative rods | + | + | + | + | + | + | + | + | + | + |
| Fungi |
| Aspergillus spp. | + | + | + | + | + | + | + | + | + | + |
| Mucor spp. | + | + | + | + | + | + | + | + | + | + |
| Rhizopus spp. | - | + | - | - | + | - | + | + | - | + |
| Penicillium spp. | + | + | - | - | + | - | + | - | - | - |
| Exophiala spp. | + | - | - | - | - | - | - | - | - | - |
| Absidia spp. | - | - | - | - | + | - | - | - | - | - |
MH1: Maternity home 1; MH2: Maternity home 2; MH3: Maternity home 3; MH4: Maternity home 4; MH5: Maternity home 5; GW: General ward; PW: Private ward; +: Present; -: Absent
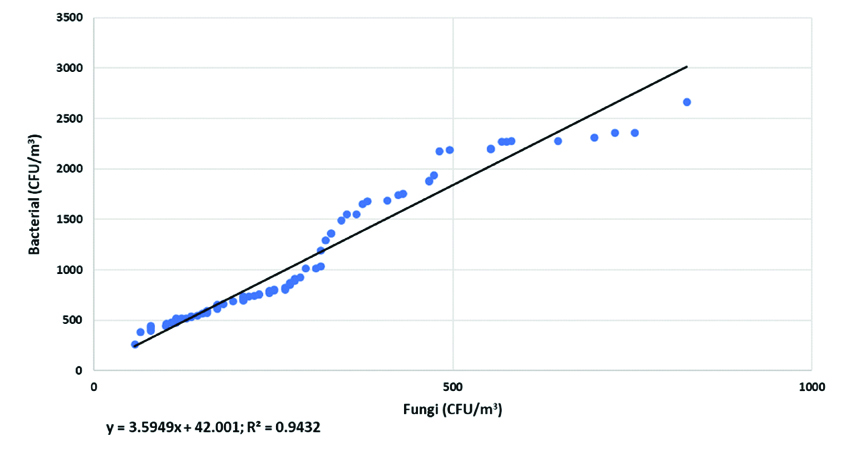
The Spearman’s test shows a positive correlation (r=0.971, n=79, p<0.001) between the concentration of bacteria and fungi. The correlation between bacteria and fungi concentration (R2=0.9432, n=79) was also demonstrated by the regression model [Table/Fig-3]. This depicts the strong correlation between the concentration of bacteria and fungi and thus the concentration of one affects the concentration of other directly.
Relationship between bacterial and fungal concentration at private maternity homes.
Discussion
Assessment of the indoor air quality in context to microbial contamination is required for both to quote the health risk and enable us to create the standard limits for indoor air microbial control. The estimation of the bacterial and fungal concentration of indoor air of five private maternity home by passive air sampling procedure ranged between 259-2665 CFU/m3 for bacteria and 79-826 CFU/m3 for fungi (irrespective of time of sampling). The study conducted in the hospitals of Egypt and teaching hospital wards of Jimma University also showed the similar result whereas the similar study conducted in hospitals of South Chennai showed low count of both bacteria and fungi [12,14,15]. The concentration of bacteria and fungi were considerably different for each private maternity home. These variations can be rendered because of the location, surrounding, hygiene and maintenance procedure of maternity homes. The value of bacterial load, as well as fungi load, was low in GW as compare to PW in the morning. Whereas, the pattern of the bacteria and fungi load in the afternoon was opposite to that of the morning. The reason associated with this is the presence of the family members near the patient in the PW in the morning whereas people often gather more in the afternoon in GW.
The previous studies have also reported same bacterial and fungal genera [14-16]. Many studies attempted the assessment of the load of microbial contamination by passive sampling; certain studies provide the significant correlation result and other inconsistent results [5,17,18]. The bacterial species that have isolated are commonly associated with the skin (humans) and mucosa, thus indicate the bacteria contamination in the air is due to the presence of human [19]. The fungus, such as Aspergillus spp., Penicillium spp., and Mucor spp., are acclaimed to be opportunistic pathogen for humans and also associated with symptoms of clinical issues such as asthma, chronic exudative inflammation, and obstructive pulmonary disease [20-22].
There is non-availability of the uniform international standards for optimal range of microbial load. The other countries have their standards such as the sanitary standard by European Commission, Italian Swiss Hospital Association Standards and (PN 89/Z-04008/08) Polish Standards for estimating the significance of the concentration [23-25]. Thus, the obtained result for the concentration of the microbes (bacteria and fungi) was compared with Fisher standards of medical wards which suggest 450-750 CFU/m3 of microbial count to be in acceptable range [11]. However, if the value of microbial count is more than 750 CFU/m3 it falls in the non-acceptable and contaminated range. Our future study will address to check the drug susceptibility test of the isolated strains against different antibiotics. Additionally, there is need to construct the maternity homes by adopting the specific guidelines for constructing the medical facilities such AIA (American Institute of Architects) guidelines can be followed [26].
Limitation
Due to the limitation of resources, the small sample size analysis was done. Due to no funding, passive air sampling was done as active air sampling requires specific instrumentation. Moreover, lack of instrument facilities limited us to for incomplete and partial identification of bacteria. Also, restricted us from identifying fungal species and MDR pattern analysis of all the isolates. In future, the long-term study will be conducted for reinforcing the findings of the study. This preliminary analysis is an initiation for future studies across the entire and nearby regions of the Moga, Punjab, India.
Conclusion
The present study enlightens us about the presence of the air-microbes in the maternity homes that can be potent to induce nosocomial infection. The high concentration of bacteria and fungi indicates that there is a need for the intrusion to regulate the growth and development of microbes in the hospitals and maternity homes. The strict hygiene protocol, proper ventilation, and regular microbial assessment is required to reduce the hazard by these airborne microbes (MDR and pathogenic). Further, the awareness and education is required to the workers of healthcare facilities to maintain environment hygiene and routine assessment by the higher authorities is required.
Authors’ Contribution
HK, and RP participated in the conception and design of the study. AK, BK, DSD, RKG, UZ and AK collected data in the field, performed experiment. HK, DSD performed the statistical analysis and drafted the manuscript. RP coordinated the study and provide the external quality assurance for the data. HK, DSD, and RKG revised the paper critically for substantial intellectual content. All authors read and approved the final manuscript.
MH1: Maternity home 1; MH2: Maternity home 2; MH3: Maternity home 3; MH4: Maternity home 4; MH5: Maternity home 5; *Optimal (Range-0-450; †Acceptable (range-451-750); ‡Not Acceptable (range->751); H: Humidity; T: Temperature
MH1: Maternity home 1; MH2: Maternity home 2; MH3: Maternity home 3; MH4: Maternity home 4; MH5: Maternity home 5; GW: General ward; PW: Private ward; +: Present; -: Absent