During pregnancy, protein excretion is increased due to a decrease in the proximal tubule reabsorption of Low-Molecular-Weight (LMW) proteins filtered by the glomerulus. Importantly, even healthy pregnant women can register proteinuria of >300 mg/dL [1].
Quantification of proteinuria in a 24 hours urine sample is mandatory in the comprehensive assessment of women with hypertensive disorders during pregnancy and, in some cases, it is necessary to establish the diagnosis of preeclampsia and its severity [2,3].
Significant proteinuria is defined by the National High Blood Pressure Education Program (NHBPEP) working group as an excretion of >300 mg of protein in a 24 hours urine sample. The same working group also states that proteinuria of 2 gm/day or greater involves increased disease severity, and this figure is set as a criterion for defining severe preeclampsia [4]. In contrast, the American College of Obstetrics and Gynaecology (ACOG) establishes a proteinuria of 5 gm/day or more for the diagnosis of severe preeclampsia [5].
Quantification of protein in a 24 hours urine collection is considered the gold standard for determining the presence of significant proteinuria in pregnancy [6]. However, the collection of urine every 24 hours is uncomfortable for the patient, time-consuming, and is subject to errors due to inadequate collection, delays the diagnosis for at least 24 hours, and it has been reported that fewer than one half of the sick women hospitalised with diagnosis of preeclampsia have a 24 hours urine collection. Due to the disadvantage of collecting 24 hours urine, other options have been proposed for the diagnosis of significant proteinuria in pregnancy. These other tests are fast and reliable, for example, the use of labstix or a test strip, measurement of proteinuria in short periods of urine collection, and the protein/urine creatinine ratio [7-9].
Protein three-dimensional conformation is the result of unfolded polypeptides that goes through a molecular folding process for which several models have been proposed. The misfolded proteins produce amorphous aggregation, a phenomenon determined by the melting temperature (Tm) [10]. Some protein misfolding diseases include Alzheimer’s, Parkinson’s and some neurodegenerative diseases [11]. Interestingly, preeclampsia is characterised by the increased excretion of misfolding protein, attracted by a Diazo dye enominated congo red [12]. By using an unbiased mass spectrometry proteomic profiling approach, the group of Buhimschi IA et al., discovered that urine of women with severe preeclampsia displayed a characteristic set of proteomic biomarkers consisting of nonrandom proteoforms of SERPINA1 and albumin [13]. SERPINA1 fragments have the propensity to misfold and aggregate into supramolecular structures [14], thus showing congophilia, a characteristic of protein misfolding [15]. The aim of this study was to compare congo red staining kit versus a urocolor test to determine proteinuria in patients with preeclampsia, looking for a more specific option with good correlation to the 24 hours urine collection test.
Materials and Methods
This was a prospective, descriptive, cross-sectional, pilot study conducted at the “Mónica Pretelini Sáenz” Maternal-Perinatal Hospital (HMPMPS), Health Institute of the State of Mexico (ISEM), Toluca from August 2010 to July 2011. The sample was set with a convenience non-probability sampling, based on the congo red tests available. Fifty pregnant women with >20 and <41 weeks gestation or in immediate puerperium exhibiting the clinical criteria of preeclampsia were enrolled in the study. The diagnosis of preeclampsia was consistent with the recommendations of the ACOG [5]. Women with a history of preeclampsia, Urinary Tract Infection (UTI), kidney disease, or gestational hypertension were excluded from the study. Patients with incomplete medical files were discarded from the final analysis. The present study was approved by the Ethics Committee of the HMPMPS (Code: EI/136) and was conducted in accordance with the Helsinki Declaration (Fortaleza, Brazil). Written informed consent was obtained from all patients who participated in the study.
General Information
The primary source of information was collected through the review of medical records and taking into account the following: age; educational level; factors associated with a gestational hypertensive disorder; number of sexual partners; prenatal care; type of delivery of the infant; main indication for cesarean section, and maternal and perinatal outcomes.
Congo Red
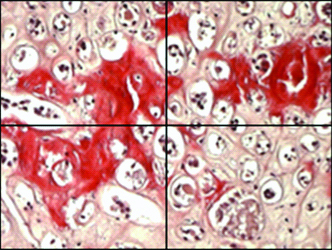
The Highman’s method was adjusted using the semiquantitative Congo Red Kit (catalogue #101641; Merck Millipore, USA) [16]. The Congo red staining principle is based on the formation of hydrogen bridge bonds with the carbohydrate component of the substrate. Congo red is an anionic dye and is capable of depositing itself in amyloid fibrils, which then exhibit a conspicuous dichroism under polarised light. The tissue stained with Congo red appears orange-red under the transmitted-light microscope; under polarised light; however, the amyloid deposits show up as brilliant green double-refraction images against a dark background. The staining process was conducted by the Clinical Laboratory Service, taking an average of 12 minutes. The reading was performed by two chemical pharmacobiologists and an expert in anatomopathology. Each slide was divided in four visual fields at the moment of the microscopic visualisation to give a report based on the positive quadrants (fields).
Process
In Highman’s method, the staining was done with an alcoholic solution (in present case 2% isopropyl alcohol was used), followed by a differentiation with an alkaline solution (KOH). In the present process the urinary sediment was used as a specimen to study and therefore steps of the process were minimised.
The sample was collected after a centrifugation for 3 minutes, and then it was fixed with heat. The next step was staining in congo red solution, followed by rinsing and drying. The interpretation was estimating its presence and the distribution in fields (quadrants) of vision in the microscope [Table/Fig-1]. It was reported as a positive result to dark blue nuclei whose center (amyloid) was pink-red pigmented and the periphery metachromasia green when using polarised light. Simultaneously, the 24 hours urine collection, and two measurements with urocolor 11 test strips within the first 6 hours, using a chromatic scale were also performed.
Exemplification of the microscopic slide division in quadrants.
Statistical Analysis
Quantitative variables were expressed as mean±Standard Deviation (SD) and qualitative variables in frequency and percentage. The normality hypothesis was tested using the Kolmogorov-Smirnov test. Differences among nominal conditions were evaluated by the Mann-Whitney U-test. The intra and interobserver Kappa index were calculated for the identification of positive congophilia. In all cases, p≤0.05 was considered statistically significant. All tests were performed with the SPSS version 23.0 statistical software program (IBM SPPS 23, USA).
Results
General Characteristics
Considering 436 hospital admissions (0.88 admissions/day) into the Obstetrics Intensive Care Unit (OICU) during the study frame time, preeclampsia represented a total of 223 cases (51.14%) (0.44 admissions/day). From all this population only 50 women were selected. Mean age was 27.4±7.2 years, with an average hospital stay of 3.6±1.5 days and with three organ dysfunctions. The [Table/Fig-2] shows the general characteristics of the studied population.
General characteristics of the patients.
| Variable | Value |
|---|
| Age (years) | 27.4±7.2 |
| Gestational age (weeks) | 33.96±2.5 |
| Mean arterial pressure (mmHg) | 111.6±24.3 |
| Proteinuria (mg/dL) | 3076.2±1677.6 |
| Stay in the obstetrical ICU (days) | 3.6±1.5 |
ICU: Intensive care unit
In relation to the obstetrical data, 1 (28%) were primiparous and the average number of pregnancies was 2.72. Women were found at an obstetric gestational age of 33.96 weeks (early preeclampsia) and 45 (90%) had risk factors. The [Table/Fig-3] shows the distribution by age. Analysis of organ dysfunction was based on the Sequential Organ Failure Assessment (SOFA) classification and the main organ dysfunctions are described in [Table/Fig-4] [17]. At the moment of hospital discharge, >96% of patients had experienced remission of all dysfunctions. 26 (52%) of all women had fetal compromise. Fetal outcomes are described in [Table/Fig-5].
Distribution of patients per age range.
| Age range (Years) | n (Percentage) |
|---|
| 15-20 | 9 (18%) |
| 21-25 | 12 (24%) |
| 26-30 | 14 (28%) |
| 31-35 | 6 (12%) |
| 36-40 | 7 (14%) |
| ≥40 years | 2 (4%) |
| Total | 50 (100%) |
| Organ failure | n (Percentage) |
|---|
| Renal | 50 (100%) |
| Haematological | 31 (62%) |
| Hepatic | 29 (58%) |
| Cardiovascular | 28 (56%) |
| Neurological | 13 (26%) |
| Respiratory dysfunction | 7 (14%) |
| Outcome | Unit |
|---|
| APGAR score at 5 minutes (mean±SD) | 7.4±1.8 |
| Fetal weight (gm) (mean±SD) | 1951.9±742.5 |
| Intrauterine growth restriction (cases) | 12 (24%) |
| Cesarean section (cases) | 44 (88%) |
SD: Standard deviation
Congo Red
Congophilia was present in two fields in 12 (24%), in three fields 34% (n=17), and in four fields, in 21 (42%). The intraobserver Kappa index was 0.87 (expected, 0.5) and the interobserver Kappa index was 0.740 {Standard Error (SE): 0.067; 95% Confidence Interval (95% CI), 0608-0872}.
In the whole population mean proteinuria value was 3,076.2 mg. In 4 (8%) protein determination was <1,000 mg, while in 10 (20%) this fell within the range of 1,001-2,000, 12 (24%) within the range of 2,001-3,000 mg, 15 (30%) within the nephrotic range between 3,001 and 4,000 mg, 4 (8%) exhibited between 4,001 and 5,000 mg and over 5,000 mg, 5 (10%). Visualising this information. The [Table/Fig-6] shows the mean proteinuria values per number of positive congo red fields and displays the information for urocolor 11 test strips [Table/Fig-7]. Contrastingly, although that 100% of cases were positive for congo red test none was 4+ positive in the urocolor 11 strip despite signs of nephrotic-range proteinuria [Table/Fig-8].
Proteinuria values per number of congo red positive fields.
| Congo red fields | N | Mean | Standard deviation | Error | 95% Confidence interval for the mean | Minimum | Maximum |
|---|
| Lower limit | Upper limit |
|---|
| 2 | 12 | 1366.4 | 375.0 | 108.2 | 1128.0 | 1604.7 | 934.2 | 1892.0 |
| 3 | 17 | 2664.5 | 471.0 | 114.2 | 2422.3 | 2906.7 | 1490.0 | 3349.7 |
| 4 | 21 | 4386.7 | 1714.3 | 374.0 | 3606.3 | 5167.0 | 2763.1 | 9967.1 |
| Total | 50 | 3076.2 | 1677.6 | 237.2 | 2599.5 | 3553.0 | 934.2 | 9967.1 |
Number of urocolor positive crosses per congo red positive fields.
| Congo red positive fields | Urocolor 11 strip positive crosses | Total |
|---|
| None | One | Two | Three |
|---|
| 2 | 6 | 6 | 0 | 0 | 12 |
| 3 | 9 | 5 | 2 | 1 | 17 |
| 4 | 6 | 12 | 3 | 0 | 21 |
| Total | 21 | 23 | 5 | 1 | 50 |
Congo red positive fields, urocolor test and proteinuria in 24 hours urine collection.
| Case | Positive congo red fields in the microscope slides | Urolabstix | Proteinuria in 24 hours urine collection (mg) |
|---|
| 1+ | 2+ | 3+ | 4+ |
|---|
| 1 | 3 | 0 | 0 | 0 | 0 | 2865 |
| 2 | 2 | 0 | 0 | 0 | 0 | 1765 |
| 3 | 3 | 0 | 0 | 0 | 0 | 2716 |
| 4 | 2 | 0 | 0 | 0 | 0 | 958.4 |
| 5 | 2 | 1 | 0 | 0 | 0 | 1892 |
| 6 | 3 | 0 | 0 | 0 | 0 | 2789 |
| 7 | 4 | 0 | 0 | 0 | 0 | 3209 |
| 8 | 4 | 1 | 0 | 0 | 0 | 9967.1 |
| 9 | 2 | 0 | 0 | 0 | 0 | 1243.6 |
| 10 | 3 | 0 | 0 | 0 | 0 | 2345.7 |
| 11 | 4 | 1 | 0 | 0 | 0 | 3522.2 |
| 12 | 3 | 0 | 0 | 0 | 0 | 2891 |
| 13 | 2 | 1 | 0 | 0 | 0 | 934.2 |
| 14 | 4 | 0 | 0 | 0 | 0 | 6540 |
| 15 | 3 | 1 | 0 | 0 | 0 | 3240 |
| 16 | 4 | 0 | 1 | 0 | 0 | 3819 |
| 17 | 3 | 0 | 1 | 0 | 0 | 2782 |
| 18 | 4 | 1 | 0 | 0 | 0 | 3276 |
| 19 | 4 | 0 | 1 | 0 | 0 | 3202.1 |
| 20 | 4 | 1 | 0 | 0 | 0 | 3298.1 |
| 21 | 3 | 0 | 0 | 1 | 0 | 2952.4 |
| 22 | 2 | 0 | 0 | 0 | 0 | 1856 |
| 23 | 4 | 1 | 0 | 0 | 0 | 4563.2 |
| 24 | 4 | 0 | 1 | 0 | 0 | 3248.5 |
| 25 | 4 | 1 | 0 | 0 | 0 | 2763.1 |
| 26 | 3 | 0 | 1 | 0 | 0 | 2840.2 |
| 27 | 4 | 1 | 0 | 0 | 0 | 3329 |
| 28 | 2 | 1 | 0 | 0 | 0 | 1455.3 |
| 29 | 3 | 1 | 0 | 0 | 0 | 3345.3 |
| 30 | 4 | 1 | 0 | 0 | 0 | 4356.2 |
| 31 | 3 | 1 | 0 | 0 | 0 | 2314.6 |
| 32 | 2 | 1 | 0 | 0 | 0 | 1356 |
| 33 | 4 | 1 | 0 | 0 | 0 | 6577 |
| 34 | 3 | 1 | 0 | 0 | 0 | 2344 |
| 35 | 4 | 0 | 0 | 0 | 0 | 4562 |
| 36 | 4 | 1 | 0 | 0 | 0 | 3325 |
| 37 | 2 | 1 | 0 | 0 | 0 | 980 |
| 38 | 2 | 1 | 0 | 0 | 0 | 1778 |
| 39 | 4 | 1 | 0 | 0 | 0 | 3459 |
| 40 | 4 | 1 | 0 | 0 | 0 | 4356 |
| 41 | 4 | 0 | 0 | 0 | 0 | 5890 |
| 42 | 3 | 1 | 0 | 0 | 0 | 2347.9 |
| 43 | 4 | 0 | 0 | 0 | 0 | 3298 |
| 44 | 4 | 0 | 0 | 0 | 0 | 5560.2 |
| 45 | 3 | 0 | 0 | 0 | 0 | 1490 |
| 46 | 3 | 0 | 0 | 0 | 0 | 2210.1 |
| 47 | 2 | 0 | 0 | 0 | 0 | 1198.1 |
| 48 | 2 | 0 | 0 | 0 | 0 | 980.2 |
| 49 | 3 | 0 | 0 | 0 | 0 | 3349.7 |
| 50 | 3 | 0 | 0 | 0 | 0 | 2474 |
Kolmogorov-Smirnov normality test was used and evidenced a non-Gaussian distribution for proteinuria per group of red congo positive identification. In view of the results, Mann-Whitney U-test was used to compare the proteinuria values [Table/Fig-9].
Comparison of proteinuria per congo red positive fields.
| Groups of comparison per congo red positive fields | Mann-Whitney U-test | W of Wilcoxon | Z | Sig. |
|---|
| 2 vs. 3 | 4 | 82 | -4.34 | ≤0.001 |
| 2 vs. 4 | 0.0 | 78 | -4.715 | ≤0.001 |
| 3 vs. 4 | 27 | 180 | -4.448 | ≤0.001 |
Sig: Significance
On the other hand, the mean proteinuria values per number of crosses in the urocolor strip are shown in [Table/Fig-10]. Immediately stands out the fact that the proteinuria did not increase in relation to the crosses and the comparison was not significant [Table/Fig-11].
Mean proteinuria values per number of crosses in the Urocolor strip.
| Number of crosses | N | Mean | Standard deviation | Error | 95% Confidence interval for the mean | Minimum | Maximum |
|---|
| Lower limit | Upper limit |
|---|
| none | 21 | 2866.2 | 1595.8 | 348.2 | 2139.8 | 3592.6 | 958.4 | 6540.0 |
| 1 | 23 | 3251.2 | 1960.7 | 408.8 | 2403.3 | 4099.1 | 934.2 | 9967.1 |
| 2 | 5 | 3178.3 | 414.5 | 185.3 | 2663.6 | 3693.0 | 2782.0 | 3819.0 |
| 3 | 1 | 2952.4 | . | . | . | . | 2952.4 | 2952.4 |
| Total | 50 | 3076.2 | 1677.6 | 237.2 | 2599.5 | 3553.0 | 934.2 | 9967.1 |
Comparison of proteinuria per urocolor positive crosses.
| Groups of comparison per urocolor positive crosses | Mann-Whitney U-test | W of Wilcoxon | Z | Sig. |
|---|
| None vs. 1 | 203 | 434 | -0.905 | 0.366 |
| None vs. 2 | 36 | 267 | 1.073 | 0.283 |
| None vs. 3 | 7 | 238 | -0.552 | 0.581 |
| 1 vs. 2 | 56 | 332 | -0.090 | 0.928 |
| 1 vs. 3 | 10 | 11 | -0.217 | 0.828 |
| 2 vs. 3 | 2 | 3 | -0.293 | 0.770 |
Sig: Significance
Discussion
As noted, the percentage of caesarean sections exceeded the expected international goals, with 44 cases (88%). This is a national trend that has been widely discussed [18,19].
Today, many hospitals include the use of urine dipstick in the comprehensive evaluation of women with hypertensive disorders of pregnancy because it comprises part of international criteria for preeclampsia [20,21]. The urine dipstick is inexpensive and easy to use, and the result of the application of this resource is that it determines an approximate protein concentration, but not an absolute value. The patient’s hydration status can influence the result; in fact, a false-negative result may occur in urine samples with a density of <1.010, or in the presence of high salt concentrations, with a very acidic proteinuria pH or proteinuria not due to the presence of albumin. All of these factors render the urine dipstick poorly correlated with the 24 hours proteinuria determination, leading to a low diagnostic performance as an indicator of significant proteinuria [22]. The analysis of several studies that have investigated the reliability of the urine dipstick have found low sensitivity and specificity, resulting in a diagnosis of preeclampsia that is poorly sustained or underdiagnosed [23,24]. This last sentence is accordance with our results as in some severe cases of proteinuria, identified both by 24 hours urine collection and congo red, the urine dipstick was negative. Therefore, with the urocolor strip there was poor association with disease severity ranges.
Although, determination of proteinuria by dipstick is deficient compared with the proteinuria value in the 24 hours urine sample [25], it is widely used. Even more, the absence of proteinuria on dipstick testing may have ruled out the presence of significant proteinuria in patients. Another alternative for the presence-of-proteinuria feature has been collecting urine for short periods (8 or 12 hours), which shares limitations and problems with the collection of 24 hours urine [26]. In this current manuscript, 38% of the patients had proteinuria ≥3 gm/dL, illustrating the severity of present cases.
In a pilot study based on the congophilia test in urine, Buhimschi IA et al., found that 4/35 (11%) of asymptomatic women were positive for congo red prior to the development of preeclampsia [12]. These findings indicate the possibility of developing a test to predict and diagnose preeclampsia that only requires a few drops of urine, according to the author.
A previous study revealed a Detection Rate (DR) of 93% and a False-Positive Rate (FPR) of 4%. However, with first-trimester urine samples, DR was 33.3, 16.1, and 20% for early, late, and all cases of preeclampsia, respectively, with a 12.8% FPR. The Odds Ratio (OR) for preeclampsia was superior to Body Mass Index (BMI), and Mean Arterial Blood Pressure (MAP), but inferior to previous preeclampsia and black ethnicity. In the first trimester, the Congo Red urine test adds accuracy for preeclampsia prediction in obese, black women who had previous preeclampsia and above-average mean arterial pressure [27].
In the present pilot study, the positivity with congo red was 100%, but we have to be aware that the observed variability in the positive fields is an issue that can be used for quality and interobserver analysis.
Recently, Parikh L et al., in a study with 101 women over 14 weeks gestation undergoing evaluation for preeclampsia and diabetes proved that the Congo Red Retention (CRR) was not increased in diabetic gravidas without preeclampsia even in the presence of baseline nephropathy. They also determined that a CRR over 15% correctly classified all women with preeclampsia and adverse pregnancy outcomes [28].
It is clear from this study that there is an increase in proteinuria values, with the Congo Red staining from two to three positive fields in 1298.1 mg and 1722.2 mg from three to four positive fields. Furthermore, the statistical comparison between 2 vs. 3, 2 vs. 4 and 3 vs. 4 congo red positive fields at the microscope slide visualisation reached significant difference (p≤0.001) en relation to the mean proteinuria values for each one, contrasting with the lack of significance with the urocolor test, an expected result due to the heterogeneous proteinuria increase per number of positive crosses in the strip.
The cost of the congo red it is an important issue. Although, it is more expensive than the urocolor tests available in the market, as one can infer from the statistical analysis, the second option does not offer congruent data with the proteinuria values, thus putting patients at risk of a misdiagnosis and incorrect treatment. A handicap of the CRR is that this technique might not be able to differentiate between autoimmune diseases like lupus nephritis and a hypertensive disorder of pregnancy, and this condition applies for other kits available in the market [29-31].
Preeclampsia is still one of leading causes of maternal mortality worldwide; unfortunately, reliable and economically affordable biomarkers to predict it are elusive [32]. Herein, combining biomarkers may improve the prediction of pregnancy hypertension in the early stages of gestation and we believe CRR could add to the positive predictive value of a set of tests to diagnose preeclampsia at an early stage [33].
Despite the difficulty to explore the congophilia with the selected kit, the results lead us to think about the possibility of designing a kit or a test strip using congo red to determine proteinuria at low cost, using it at home and facilitate the early detection of a possible obstetric complication. In fact, Jonas SM et al., have developed a smart phone-based diagnostic for preeclampsia administering congo red [34]. Further research is required to explore the use of congophilia as a common diagnosis test of proteinuria in pregnancy.
Limitation
A clear limitation of this study is that it was not a randomised study; also, a safety matter of concern is that the congo red stain might be carcinogenic. Finally, a second step to contrast this test with a control group and different techniques to quantify proteinuria was not possible to perform as the used kit was discarded from the Mexican market.
Conclusion
This study confirms that women with preeclampsia and chronic kidney disease without preeclampsia have elevated congophilia urine levels. In this regard, the CRR interpreted a positive fields in the slide which shows a direct relationship with the proteinuria levels. Thus, the congo red test point represents a new diagnostic tool for the clinician working in the obstetrics area, who are looking for a quick and highly reliable test. This is a test that can be performed in any context, even in third-world countries, and the results after 10-15 minutes are obtained. In summary, the overall assessment of the misfolded protein load by congo red staining is expected to be a simple, efficient and highly reproducible diagnostic test. The results are very encouraging to continue a new line of research. By contrast, the urocolor test is not reliable to make a decision for renal injury in pregnancy/puerperium.