Sebaceous Gland Hyperplasia at Episiotomy Site: A Causal or an Incidental Association
Uma Kumar1, Annu Nanda2, Sangeeta Lamba3
1 Specialist, Department of Pathology, ESI Hospital, Rohini, Delhi, India.
2 Professor, Department of Pathology, ESI Hospital, Rohini, Delhi, India.
3 Senior Specialist and Head, Department of Pathology, ESI Hospital, Rohini, Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Annu Nanda, Professor, Department of Pathology, ESI Hospital, Rohini, Delhi-110089, India.
E-mail: dr.uma.path@gmail.com, annunanda@rediffmail.com
Sebaceous Gland Hyperplasia (SGH) is a benign skin lesion that commonly occurs on the face of elderly patients. Vulva is an extremely rare site to be reported. Only 20 cases of vulvar SGH have been reported in literature. We report a case of vulvar SGH associated with episiotomy surgery.
A 22-year-old female presented in gynaecology Out Patient Department (OPD) with complaints of vulvar swelling for three months. She had spontaneous vaginal delivery one year ago during which mediolateral episiotomy was performed. A clinical diagnosis of fibroepithelial polyp was made and the lesion was excised under local anaesthesia. Haematoxylin and eosin stained sections showed numerous sebaceous glands in a lobular arrangement associated with an infundibulum. A diagnosis of SGH was rendered on histopathology. Though, a rarity, vulvar SGH should be kept in mind while dealing with vulvar polypoidal lesions.
Benign skin, Polypoidal lesions, Vulva
Case Report
A 22-year-old female presented in gynaecology OPD with complaints of vulvar swelling for three months. She had spontaneous vaginal delivery one year ago during which mediolateral episiotomy was performed. Her episiotomy had been infected, for which she took antibiotics. Her medical and surgical history was unremarkable. On local examination, a tender polypoidal lesion measuring 1.5×1.0×1.0 cm was seen on the medial surface of right labium minus, just superior to the episiotomy scar. Overlying skin was unremarkable. Her routine per speculum and per vaginal examination was normal. The biochemical and haematological investigations were within normal limits. A clinical diagnosis of fibroepithelial polyp was made and the lesion was excised under local anaesthesia.
Gross examination revealed a skin covered polypoidal tissue measuring 1.5×1.0×0.8 cm. Cut section was solid, firm and grey white.
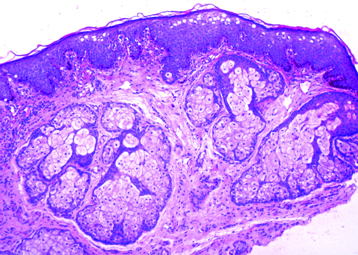
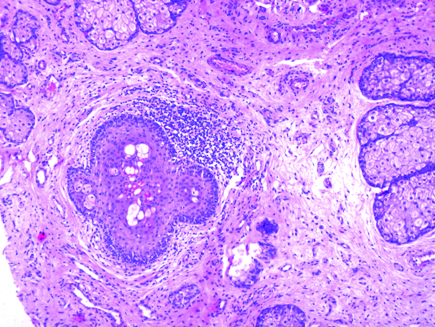
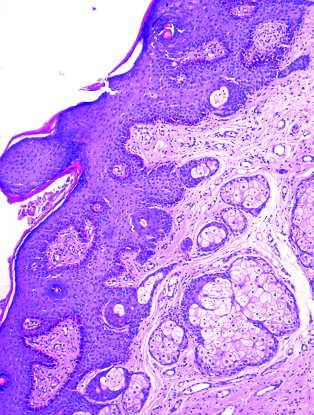
Haematoxylin and eosin stained sections showed numerous sebaceous glands in a lobular arrangement associated with an infundibulum [Table/Fig-1]. The cells were predominantly mature sebocytes with peripheral one to two layers of germinative layer. The cells had foamy cytoplasm, centrally placed nucleous and inconspicuous nucleoli. Mild mononuclear inflammatory infiltrate was also noted around infundibulum [Table/Fig-2]. Overlying epithelium showed basal hyperpigmentation and mild lentiginous hyperplasia of basal melanocytes [Table/Fig-3]. No mitosis or nuclear atypia was noted. Based on these features a diagnosis of SGH of vulva was rendered. On six months follow-up, there is no recurrence.
Section showing numerous sebaceous glands in a lobular arrangement associated with an infundibulum (H&E 4X)
Section showing mononuclear cell infiltrate around infundibulum (H&E 10X)
Overlying epidermis showing basal hyperpigmentation and lentiginous hyperplasia of basal melanocytes (H&E 4X)
Discussion
The SGH is a benign skin lesion that commonly occurs on the face, forehead and cheek of elderly patients [1]. However, there are a few case reports of this lesion on chest, areola, mouth, scrotum, foreskin, shaft of penis and vulva [2]. Vulva is an extremely rare site of occurrence. Rocamora A et al., reported the first case of SGH with polypoidal lesions presented on both labia majora and mons veneris [1]. Since then 20 cases have been described in literature to the best of our knowledge, this is the 21st case [3,4]. No case of vulvar SGH was noted in a study conducted by Baker GM et al. over a period of 32 years on vulvar adnexal lesions [5].
The SGH belongs to the group of epidermal tumors with sebaceous differentiation [6]. The typical lesion of SGH is single or multiple, soft, umbilicated papule 2-3 mm in diameter on the face of an elderly person [7]. Patients usually present with the complaints of vulvar swelling, vulvar burning and pruritics [4,7]. This patient presented with vulvar swelling for three months. Contrary to facial lesion the vulvar lesions present in women of reproductive age and with variable clinical presentation from raised or elevated lesion to a large polypoidal mass, similar to the present case [2,4].
Many factors are hypothesised to be involved in the pathogenesis of SGH which include prolonged ultraviolet radiation exposure and previous surgery [3]. Few studies suggest an inflammatory stimulation of sebaceous glands [8]. In the present case, there was a history of episiotomy surgery near the lesion and the biopsy showed non specific chronic inflammation, which could have led to sebaceous gland proliferation. There is no universally accepted criteria for histological diagnosis of SGH; however, Barnhill RL and Crowson AN defined SGH as presence of more than four sebaceous lobules attached to the infundibulum of each pilosebaceous unit [9]. Few lobes show more than one peripheral row of undifferentiated germinative cells. Sebaceous cells are positive for Epithelial Membrane Antigen (EMA) and the peripheral germinative cells are positive for CK5/6, p63 and androgen receptor [3].
Clinically, vulvar SGH can mimic achrochordon, fibroma, fibromyoma, lipoma, epidermal inclusion cyst, pyogenic granuloma, bartholin cyst [3,8]. In present case, a clinical diagnosis of fibroepithelial polyp was made. Histopathologically, SGH should be differentiated from other lesions showing sebaceous differentiation like Fordyce’s granules, nevus sebaceous of Jadassoh, sebaceous adenoma, folliculosebaceous cystic hamartoma, sebaceous gland carcinoma and sebaceoma [3,4].
Fordyce’s granules are ectopic sebaceous glands present on buccal mucosa, upper and lower labial mucosa, lips and retromolar areas [8]. These are seen as small yellow papules measuring 1-3 mm without central depression. Microscopically, there are ectopic sebaceous glands without attached follicles [8,10].
In nevus sebaceous of Jadassoh, sebaceous glands follow the pattern of normal sebaceous gland during different stages of life. In adults, there are large number of mature sebaceous glands with overlying papillomatous hyperplasia of the epidermis [5,6].
Sebaceous adenoma are composed of incompletely differentiated sebaceous lobules that are irregular in size and shape. Two types of cells are identified undifferentiated basaloid cells and mature sebaceous cells [4]. Folliculosebaceous cystic hamartoma consists of a folliculosebaceous proliferation with a cyst like infundibular dilation and a mesenchymal stroma with variable fibroplasia [3,4]. Sebaceous carcinoma is characterised by irregular lobules of undifferentiated cells with marked nuclear atypia and pleomorphism. Focal sebaceous differentiation can be seen [10]. Sebaceoma is composed of well circumscribed nodules of basaloid epithelial cells with islands of sebaceous cells. Mitoses can be seen but it lacks the atypia as compared to sebaceous carcinoma [5].
Treatment strategy of vulvar SGH includes cryotherapy, cauterisation, topical chemicals and surgery [7,8]. No recurrence or malignant transformation has been seen in the previously reported cases [3,4].
Conclusion
We report an extremely rare case of vulvar SGH associated with episiotomy surgery. Though, the clinical presentation of facial and vulvar lesions are different, it is important for the clinicians to recognise this entity. Sections should be examined carefully to exclude intraepithelial lesion or malignancy.
[1]. Rocamora A, Sanjonja C, Vives R, Varona C, Sebaceous gland hyperplasia of the vulva: a case reportObstet Gynecol 1986 68(3):63S-65S. [Google Scholar]
[2]. Ortiz-Rey JA, Martin-Jimenez A, Alvarez C, De La Fuente A, Sebaceous gland hyperplasia of the vulvaObstet Gynecol 2002 99(5):919-21.10.1097/00006250-200205001-0001911975959 [Google Scholar] [CrossRef] [PubMed]
[3]. Yoon G, Kim HS, Clinicopathological characterization of vulvar sebaceous gland hyperplasiaInt J Clin Exp Pathol 2016 9(7):7560-65. [Google Scholar]
[4]. Roma AA, Barry J, Pai RK, Billings SD, Sebaceous hyperplasia of the vulva: a series of cases reporting no association with the Muir-Torre syndromeInt J Gynecol Pathol 2014 33(4):437-42.10.1097/PGP.0b013e31829ff21e24901406 [Google Scholar] [CrossRef] [PubMed]
[5]. Baker GM, Selim MA, Hoang MP, Vulvar adnexal lesions. A 32-year, single institution review from Massachusetts general hospitalArch Pathol Lab Med 2013 137:1237-46.10.5858/arpa.2012-0434-OA23991738 [Google Scholar] [CrossRef] [PubMed]
[6]. Bakaris S, Kiran H, Kiran G, Sebaceous gland hyperplasia of the vulvaAust NZJ. Obstet Gynaecol 2004 44(1):75-76.10.1111/j.1479-828X.2004.00165.x15089875 [Google Scholar] [CrossRef] [PubMed]
[7]. Dieh AP, Jones AS, An unusual presentation of sebaceous gland hyperplasia of the vulvaJ Obstet Gynecol 2005 25:729-30.10.1080/0144361050030749016263561 [Google Scholar] [CrossRef] [PubMed]
[8]. Al-Daraji WI, Wagner B, Ali RB, McDonagh AJ, Sebaceous hyperplasia of the vulva: a clinicopathological case report with a review of literatureJ Clin Pathol 2007 60(7):835-7.10.1136/jcp.2005.03355517513513 [Google Scholar] [CrossRef] [PubMed]
[9]. Barnhill RL, Crowson AN, Textbook of dermatopathology 2004 2nd edNew YorkThe McGraw-Hill Companies [Google Scholar]
[10]. Malliah R, Gilhooly P, Lambert WC, Heller DS, Sebaceous hyperplasia of the vulva: case report and review of the literatureJ Low Genit Tract Dis 2006 10(1):55-57.10.1097/01.lgt.0000194825.78244.20 [Google Scholar] [CrossRef]